International Journal of Virology and AIDS
Hepatocellular Carcinoma in HIV-Infected Patients: Clinical Characteristics and Prognostic Factors
Antonio Diaz-SAnchez1, Pilar Miralles2,3, Ana Matilla3-5, Teresa AldAmiz-Echevarria2,3, OscarNunez, Ana Carrero2,3, Beatriz Merino3-5, Cristina Diez2,3, Rafael Banares3-6, Gerardo Clemente3-5 and Juan Berenguer2,3*
1Servicio de Aparato Digestivo, Hospital Universitario del Sureste, Arganda del Rey, Spain
2Unidad de Enfermedades Infecciosas/VIH, Hospital General Universitario Gregorio Maranon, Spain
3Instituto de Investigacion Sanitaria Gregorio Maranon (IiSGM), Spain
4Unidad de Hepatologia, Hospital General Universitario Gregorio Maranon, Spain
5Centro de Investigacion Biomedicaen Red en el Area TemAtica de Enfermedades HepAticas y Digestivas (CIBEREHD), Spain
6Facultad de Medicina, Universidad Complutense de Madrid, Spain
*Corresponding author: Juan Berenguer, Unidad de EnfermedadesInfecciosas/VIH (4100), Hospital General Universitario Gregorio Maranon, Instituto de Investigacion Sanitaria Gregorio Maranon (IiSGM), Madrid, Spain, Tel: +34-91-586-8592; Fax: +34-91-426-5177; E-mail: jbb4@me.com
Int J Virol AIDS, IJVA-1-004, (Volume 1, Issue 1), Research Article; ISSN: 2469-567X
Received: October 30, 2014 | Accepted: December 22, 2014 | Published: December 26, 2014
Citation: Diaz-Sanchez A, Miralles P, Matilla A, Aldamiz-Echevarria T, Nunez O, et al. (2014) Hepatocellular Carcinoma in HIV-Infected Patients: Clinical Characteristics and Prognostic Factors. Int J Virol AIDS 1:004. 10.23937/2469-567X/1510004
Copyright: © 2014 Diaz-Sanchez A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We analyzed 53 HIV-infected patients with hepatocellular carcinoma (HCC) diagnosed at our institution from 1998 to 2012. All patients were coinfected with hepatitis virus (77% HCV; 12% HBV; 11% HCV+HBV), and 95% had liver cirrhosis. HCC was diagnosed under surveillance in 41% of patients. Potentially curative therapy was given to 32% of patients and palliative therapy to 30% patients. Median survival was 2 months in those diagnosed from 1998 to 2005, and 11 months in those diagnosed from 2006 to 2012; P=0.16. Survival was independently associated with HCC stage, alpha-fetoprotein serum levels, MELD score, and any treatment.
Keywords
Hepatocellular carcinoma; Human Immunodeficiency Virus; Hepatitis C Virus; Chronic Hepatitis C; Liver Cirrhosis
Introduction
Each year, more than half a million people worldwide are diagnosed with hepatocellular carcinoma (HCC), and approximately 20,000 of these casesare diagnosed in the United States [1]. Major risk factors for HCC include infection with HBV or HCV, alcoholic liver disease, and nonalcoholic fatty liver disease [1]. It is noteworthy that this neoplasm usually appears in patients with underlying cirrhosis. Since the introduction of combination antiretroviral therapy (cART), the incidence of HCC has increased steadily in HIV-infected individuals, driven primarily by HCV infection [2,4]. Recent reports indicate that HCC is an increasingly frequent cause of death among patients with HIV infection [5]. In comparison with non-HIV-infected patients, some reports suggest that at diagnosis of HCC, patients with HIV infection are younger and more frequently symptomatic with advanced tumors [6,7]. Little is known however about HCC surveillance practices in at-risk HIV-infected patients and about prognostic factors of HCC in this population group. Our objective was to assess clinical characteristics, treatment, and survival in HIV-infected patients with a particular emphasis on identification of prognostic factors.
Methods
We reviewed the Minimum Basic Data Set (MBDS) of our institution to identify all HIV-infected patients diagnosed with HCC at our institution from 1998 to 2012. The MBDS is a computerized database of clinical and administrative data generated from the medical records of discharged patients that is used in hospital management processes and clinical and epidemiological research. Patient medical records were reviewed, and data were extracted according to a protocol and recorded in a working database.
Diagnosis of HCC was based on noninvasive imaging tests or pathology findings according to well-defined criteria [8]. HCC was staged following the Barcelona/Clinic Liver Cancer (BCLC) classification [9]. We compared patients with HCC diagnosed before and after 2005, when the new HCC guidelines of the American Association for the Study of Liver Diseases were published [10]. For the purposes of the study, we considered that the diagnosis of HCC was made during surveillance when ultrasonography of the liver (with no evidence of HCC) was performed within the 12 months before the diagnosis of HCC. During the study period,a multidisciplinary liver cancercommittee met regularly to review cases, outline treatment plans, and follow outcomes of patients with liver cancer. This committee comprises hepatologists, interventional radiologists, and transplant surgeons with expertise in the diagnosis, treatment, and management of liver tumors.
Results
Patient characteristics at the time of diagnosis of HCC are shown in (Table 1). Almost 90% were male, the median age was 47 years, 82% had acquired HIV by injection of drugs, 40% had had prior AIDS defining conditions, 87% were on cART, the median CD4 cell count was 326 cells/µL, 67% had an HIV-RNA load below the limit of quantification, and 23% drank more than 50g of alcohol per day. Of note, in the second period, patients were older and had full suppression of HIV-RNA more frequently than in the first period.
![]()
Table 1: Clinical characteristics, diagnosis, and treatment of HIV-infected patients with hepatocellular carcinoma.
View Table 1
As for liver disease, all patients were coinfected with hepatitis virus (77% HCV; 12% HBV; 11% HCV+HBV), 95% had liver cirrhosis, 60% were Child-Pugh stage B or C, 50% had decompensated liver disease, and 41% had previously received Peg IFN and RBV without achieving a sustained viral response. Patients in the first period had significantly higher Model for End-Stage Liver Disease (MELD) scores than patients in the second period (Table 1).
HCC was diagnosed during surveillance in 41% of patients, with no significant differences between the periods (Table 1).
A nonsignificanttrend towards tumors with better prognostic characteristics was found in the second period in comparison with the first period (Table 1). A solitary lesion was found in 38% of patients, and lesions greater than 5 cm in diameter were found in 52% of patients. Portal vein invasion was detected in 36% of patients, and metastases in 15%. The tumor was advanced (BCLC stage C or D) in 55% of patients, and alpha-fetoprotein serum levels were above 200 ng per ml in 38% of patients.
Sixty percent of patients received treatment for HCC. Of note, patients in the second period received treatment for HCC more frequently than patients in the first period. Potentially curative therapy was given to 32%of patients and noncurative therapy to 30%of patients.
The median (IQR) duration of follow-up was 10 (1-23) months. The median (IQR) survival was 2 (1-27) months in the first period and 11 (3-23) months in the second period; P=0.16. A nonsignificant trend towards improved survival during the first 3 years after diagnosis of HCC was observed in the second period (1-year survival, 62%; 2-year survival, 37%; and 3-year survival, 28%) in comparison with the first period (1-year survival, 37%; 2-year survival, 26%; and 3-year survival, 14%). Variables associated with survival by univariate and multivariate Cox regression analysis are shown in (Table 2). Survival was independently associated with tumor-related factors (such as BCLC stage), serum alpha-fetoprotein levels, liver-related factors (such as MELD score), and any treatment (Figure 1).
![]()
Table 2: Variables associated with survival of HIV-infected patients with hepatocellular carcinoma by Cox regression analysis
View Table 2
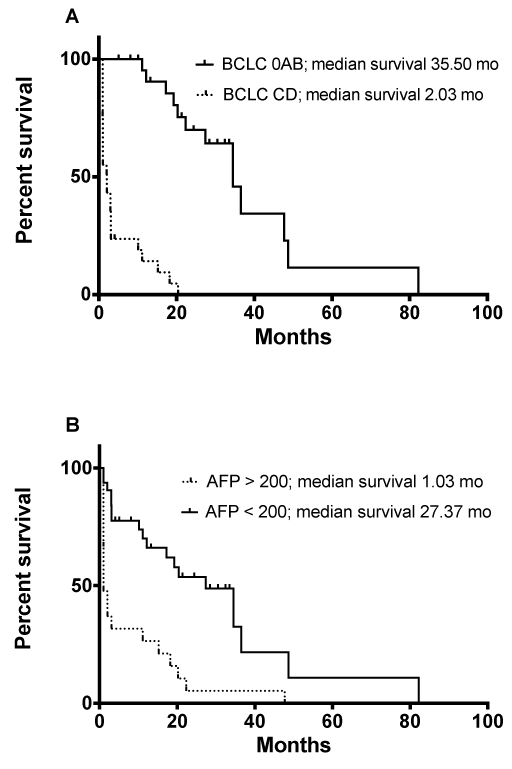
Figure 1: Kaplan-Meier estimates of survival according to the Barcelona/ Clinic Liver Cancer (BCLC) classification. The median survival was 35.50 months for nonadvanced stages (BCLC 0AB) and 2.03 months for advanced stages (BCLC CD) (Figure 1A). Kaplan-Meier estimates of survival according to serum alpha-fetoprotein (AFP) levels. The median survival was 1.03 months for patients with AFP >200ng/ml and 27.37 months for patients with AFP <200ng/ml (Figure 1B).
View Figure 1
Discussion
We analyzed the characteristics, treatment, and outcome of 53 HIV-infected patients with HCC attended in our institution over a 14-year period (1998 to 2012). HCV-related cirrhosis wasthe most frequent underlying disease, although in a large number of patients, profound immunosuppression and alcohol consumption - well known enhancers of fibrosis progression in HIV/HCV-coinfected individuals [3,11,12] - were associated with advanced liver disease.
Of note, only 40% of tumors were detected in surveillance programs, irrespective of the period analyzed; this figure differs little from data reported elsewhere [13]. It must be remembered that ours is a referral center for liver-transplantation and that a substantial proportion of patients were already diagnosed with HCC when first seen by us.We found that HCC was more frequently diagnosed using noninvasive imaging tests after 2005, thus reflecting current recommendations [1]. At diagnosis, HCC was frequently advanced, and half of the patients had decompensated liver disease; these findings are concordant with those ofother studies in this field [6,7].
A significantly higher proportion of patients received treatment for HCC after 2005. The explanation for this finding is multifactorial: in recent years, a more generalized approach to managingmalignant diseases in HIV-infected patients has been applied following the appropriate-for-stage recommendations used in the general population; in addition, new treatmentsfor HCC, such as sorafenib, have become available.
Anonsignificant trend towards increased survival was observed after 2005. However,given that the statistical power of the comparisons of mortality between the 2 periods ranged from 20% for 3-year survival and 40% for 1-year survival, a type II error could not be excluded. We found that survival was independently associated with tumor burden (BCLC stage and serum alpha-fetoprotein concentration), liver function (MELD score), and treatment of HCC. Detectable HIV-RNA was associated with an increased hazard of mortality by univariate analysis but not by multivariate analysis.
Our study is limited by its retrospective design, and by the small number of patients included. In addition, although the AASLD screening guidelines were enforced in our institution after their publication in 2005; we have no data about how diligently they were followed by clinicians. We recognize that the definition of diagnosis of HCC during surveillance used in this study (ultrasonography within the 12 months before the diagnosis of HCC) doesn't match with current recommendations that surveillance be undertaken at 6 monthly intervals [14]. However, it must be taken into consideration that the ideal surveillance interval is not known; and that a surveillance interval of 6-12 months has been proposed based on tumor doubling times [14]. Moreover, a retrospective study has reported that survival is no different in patients screened at 6 or 12 monthly intervals [15]. Nevertheless,our results reflect the experience of a single institution in which a multidisciplinary team reviewed HCC cases and outlined treatment plans. Our findings allow us to draw some conclusions that we believe are relevant for clinical practice.First, HCV-related cirrhosis was by far the most frequent underlying disease in patients with HCC, many of whom had a history of severe immunosuppression and/or alcohol consumption. Consequently, key preventive measures for HCC among HIV/HCV-coinfected individuals should include interventions that modify the natural history of HCV infection, such as antiviral therapy for HCV [16] and HIV [17] and avoidance of alcohol and injection drugs.Second, most cases of HCC were detected outside surveillance programs. Third,at the time of diagnosis, half of the patients already had decompensated liver disease and the tumor was frequently advanced. These findings highlight the need to prioritizeidentification of patients at risk of HCC,particularly those with liver cirrhosis. Patients can be identifiedaccurately using noninvasive methods such as transient elastography and serum tests [18]. Following current guidelines,patients at risk should be screened for HCC with 6-monthly liver ultrasound with or without serum alpha-fetoprotein testing [10], a procedure that is associated with significant improvements in early tumor detection, administration of curative therapy, and overall survival in patients with cirrhosis [19].
Shortening the interval for HCC screening has been proposed for HIV-infected patients with liver cirrhosis,since development of HCC could be more rapid in this group than in cirrhotic patients without HIV-infection [20]. This approach warrants further analysis in prospective studies, particularly in patients at high risk of developing liver-related events such as those with liver-stiffness values >40kPa [21,22].
Acknowledgements
This work was supported by Red de Investigacionen SIDA [AIDS Research Network] (RIS). Ref RD12/0017.J Berenguer is an investigator from the Programa de Intensificacion de la ActividadInvestigadoraen el Sistema Nacional de Salud (I3SNS). Refs INT10/009 and INT12/154.
The authors thank Thomas O’Boyle for writing assistance during the preparation of the manuscript.
References
-
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365: 1118-1127.
-
Sahasrabuddhe VV, Shiels MS, McGlynn KA, Engels EA (2012) The risk of hepatocellular carcinoma among individuals with acquired immunodeficiency syndrome in the United States. Cancer 118: 6226-6233.
-
Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, et al. (2013) The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 57: 249-257.
-
Merchante N, Merino E, López-Aldeguer J, Jover F, Delgado-Fernández M, et al. (2013) Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis 56: 143-150.
-
Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, et al. (2009) Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalite 2005 study. J Hepatol50:736-745.
-
Bourcier V, Winnock M, Ait Ahmed M, Sogni P, Pambrun E, et al. (2012) Primary liver cancer is more aggressive in HIV-HCV coinfection than in HCV infection. A prospective study (ANRS CO13 Hepavih and CO12 Cirvir). Clin Res HepatolGastroenterol 36: 214-221.
-
Berretta M, Garlassi E, Cacopardo B, Cappellani A, Guaraldi G, et al. (2011) Hepatocellular carcinoma in HIV-infected patients: check early, treat hard. Oncologist 16: 1258-1269.
-
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35: 421-430.
-
Forner A, Reig ME, de Lope CR, Bruix J (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 30: 61-74.
-
Bruix J, Sherman M (2005) Practice Guidelines Committee American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology42:1208-1236.
-
Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. (1999) Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology30:1054-1058.
-
Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, et al. (2001) Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 33: 562-569.
-
Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, et al. (2010) Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 52: 132-141.
-
Bruix J, Sherman M; American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53: 1020-1022.
-
Trevisani F, Cantarini MC, Labate AM, De Notariis S, Rapaccini G, et al. (2004) Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol 99: 1470-1476.
-
1Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, et al. (2009) Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology50:407-413.
-
Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. (2006) Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol44:47-55.
-
Castera L, Bedossa P (2011) How to assess liver fibrosis in chronic hepatitis C: serum markers or transient elastography vs. liver biopsy? Liver Int 31 Suppl 1: 13-17.
-
Singal AG, Pillai A, Tiro J (2014) Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 11: e1001624.
-
Nunnari G, Berretta M, Pinzone MR, Di Rosa M, Berretta S, et al. (2012) Hepatocellular carcinoma in HIV positive patients. Eur Rev Med PharmacolSci 16: 1257-1270.
-
Perez-Latorre L, Sanchez-Conde M, Rincon D, Miralles P, Aldamiz-Echevarria T, Carrero A, et al. (2014) Prediction of liver complications in patients with hepatitis C virus-related cirrhosis with and without HIV coinfection: comparison of hepatic venous pressure gradient and transient elastography. Clin Infect Dis 58:713-718.
-
Macias J, Camacho A, Von Wichmann MA, Lopez-Cortes LF, Ortega E, Tural C, et al. (2013) Liver stiffness measurement versus liver biopsy to predict survival and decompensations of cirrhosis among HIV/hepatitis C virus-coinfected patients. AIDS 27:2541-2549.





