International Journal of Diabetes and Clinical Research
Vitamin B12 Levels in Rat Organs - No Change Following Prolonged Treatment with Metformin
Thagaard MS, Nexo E, Greibe E*
Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark
*Corresponding author:
Eva Greibe, Department of Clinical Biochemistry, Aarhus University Hospital, Norrebrogade 44, 8000 Aarhus C, Denmark, E-mail: evagreibe@gmail.com
Int J Diabetes Clin Res, IJDCR-2-043, (Volume 2, Issue 5), Research Article; ISSN: 2377-3634
Received: August 12, 2015 | Accepted: August 27, 2015 | Published: September 02, 2015
Citation: Thagaard MS, Nexo E, Greibe E (2015) Vitamin B12 Levels in Rat Organs - No Change Following Prolonged Treatment with Metformin. Int J Diabetes Clin Res 2:043. 10.23937/2377-3634/1410043
Copyright: © 2015 Thagaard MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Few studies have explored vitamin B12 (B12) distribution in control rats and in rats treated with metformin, an anti-diabetic drug known to decrease the plasma level of B12 in humans. Here we report B12 levels in both control rats and in rats treated by two different methods of delivering metformin.
Methods: Male Wistar rats aged 16 weeks at sacrifice was treated for 6 weeks with daily subcutaneous injections of either saline (n = 10) or metformin (n = 10). We collected plasma and organs for analyses of B12, and we explored the fraction of protein-bound B12 in selected organs. In addition we examined the effect of four weeks administration of metformin (n = 10) or saline (n = 10) by intra-peritoneal osmotic pumps on levels of B12 in plasma, liver, and kidneys of rats aged 14 weeks at sacrifice.
Results: Median concentration of B12 in the 16 weeks old control rats was highest in the kidney (1350 pmol/g tissue) followed by the liver (74) and heart (72) and lowest in the brain (33), spleen (25), and lungs (10). The fraction of protein-bound B12 was around 0.30 in the kidney and 0.85 or higher in other organs. In 14 weeks old rats B12 concentration (pmol/g tissue) was around 0.7 of the concentration in 16 weeks old rats, both in liver and kidney. The relationship between kidney and liver B12 (pmol/g tissue) was similar for the two groups of rats. Neither subcutaneous nor intra-peritoneal treatment with metformin resulted in alterations in B12 distribution or concentration as compared to the controls. This was the case for both plasma and organs.
Conclusion/Interpretation: We report the kidneys of control rats to contain around two and a half fold more B12 per organ than the liver. Expressed as B12 per grams tissue, the kidney contained around fifteen fold more B12 than the liver. The total amount of B12 in these two organs increased from 14 to 16 weeks of age. Our data showed no alteration in the content or distribution of B12 upon treatment with metformin.
Keywords
Metformin, Vitamin B12, Cobalamin, Rat organs, Tissue distribution, Rat model
Introduction
As in humans, rodents depend on vitamin B12 (B12) for DNA synthesis and for the tricarboxylic acid cycle. Because of this rats and mice are often used as animal models when studying B12 metabolism [1-4]. However, systematic studies on the distribution of B12 in normal rats are scarce.
Rodents have also been used to study the effect of metformin on B12 status [1].
Metformin is an oral anti-diabetic drug used in treatment of type 2 diabetes and Polycystic Ovary Syndrome. Human studies have repeatedly reported low serum B12 as a side effect to treatment with metformin [5-7]. This in turn has led to the assumption that patients treated with metformin are at risk of developing overt B12 deficiency. Severe B12 deficiency can lead to irreversible neurological impairment and/or megaloblastic anaemia [8].
Recently it has been questioned, whether metformin treatment results in a change in the distribution of B12, so that the tissue content of the vitamin remains sufficient and only the circulating part of the vitamin decrease. This perspective has been supported by two clinical study by Obeid et al. [9] and Greibe et al. [19] and also by a recent study in rats by Greibe et al. [1].
The purpose of the present study is twofold. We aim to report B12 levels in a large number of tissues from normal rats and to study changes induced by two different methods of prolonged treatment with metformin, either with subcutaneous injections or by intra-peritoneal osmotic pumps.
Materials and Methods
Animals
Male Wistar rats (n = 20 in each study) were used. The rats were housed in pairs with 12 hours light-dark cycles and fed stock rat fodder (Altromin 1324, Altromin Spezialfutter GmbH & Co, Germany - containing 24 μg/kg B12) and water ad libitum. The study was approved by the Animal Ethics Committee and carried out at the animal lab facility at Aarhus University, Aarhus, Denmark.
Experimental design
Subcutaneous injections with metformin or saline:Two groups of male Wistar rats (n = 10 in each group, age 16 weeks at sacrifice) were treated daily with either subcutaneous (s.c.) injections with 250 mg/kg/day metformin (1,1-dimethylbiguanide hydrochloride, cat. no. D150959-5G, Sigma-Aldrich, Brondby, Denmark) dissolved into 0.5 ml 0.9% saline or with 0.5 ml 0.9% saline only (controls) in the skin of the neck for six weeks. Organs (heart, kidneys, liver, spleen, stomach, testicles, brain, small- and large-bowel, and lungs) were removed at sacrifice, and kept at -80℃ until analysed.
Blood samples were collected from the sublingual vein using lithium heparin tubes before (day 0) and from the heart at the end of the study. After collection the samples were centrifuged at 1850 x g for 9 minutes at room temperature followed by removal of plasma. Plasma was stored at -20℃ until analysis. During the study, two rats in the metformin group passed away for reasons unknown.
Metformin or saline administered by osmotic pumps: Two groups of male Wistar rats (n = 10 in each group, age 14 weeks at sacrifice) were treated with either metformin (50 mg/ml 0.9% saline, 3.6 mg/day) or 0.9% saline delivered into the intra-peritoneal cavity by osmotic pumps (ALZET 2ML4 pump, AgnTho's, Stockholm, Sweden) for four weeks. Each pump was filled under sterile conditions and according to the manufacturer's instructions. The pumps were placed within the intra-peritoneal cavity on day 1. The rats were anaesthetised using isoflorane gas and placed on a heating plate to avoid hypothermia. Under sterile conditions a small midline incision was made around one cm under the xiphoid process to accommodate the osmotic pump. After the pump had been placed, the abdominal wall was closed with 4-0 reabsorptive vicryl (Ethicon, Denmark) sutures. Afterwards the skin was closed with suture clips every 2-3 mm. Blood samples were collected as described for the first study. At the end of the four weeks, the animals were sacrificed and liver and kidneys removed and kept at -80℃ until analysed. During the study, two control rats and one metformin-treated rat passed away, most likely due to postoperative complications.
Biochemical methods
Upon removal all organs were weighed. When further processed we added 1.5 ml buffer per gram tissue and organs were homogenised on ice using a Tissue Ruptor (Qiagen, Copenhagen, Denmark). The homogenisation buffer consisted of 10 mM PIPES pH 7.4 (Sigma-Aldrich, Brondby, Denmark), 1 mM EDTA (Sigma-Aldrich, Brondby, Denmark), 3 mM MgCl2, 6H2O (Merck, Damstadt, Germany). Two tablets of protease inhibitor cocktail (Cat. no. 11697498001, Roche Diagnostics, Mannheim, Germany) were added per 50 ml. buffer. Afterwards homogenates were subjected to three freeze-thaw cycles followed by three times 10 sec. ultra sonication (MSE probe universal). After the last sonication, homogenates were centrifuged for 40.000 x g for 40 min. at 4℃ and stored at -80℃ until analysed.
B12 in plasma and tissue extracts was measured using a Cobas 6000 E system. Organ extracts were diluted 1:50, except for kidney extracts, which was diluted 1:500. The Cobas 6000 E system employs an alkaline release of B12 from its carrier proteins followed by a conversion of all B12 into cyano-B12 by addition of sodium cyanide. Ruthenium-labelled intrinsic factor binds the released B12 and excess intrinsic factor is captured by biotin-labelled B12. Streptavidin is applied in order to precipitate the intrinsic-factor-biotin-B12 complex. The amount of B12 in a sample is then inversely related to the signal measured by the system. Results are expressed as pmol per gram wet tissue or pmol per organ. To determine B12 present within circulating plasma we calculated total blood volume (TBV) and plasma volume as described by Lee et al. [11]. B12 results in plasma are given as pmol in total volume of circulating plasma and in pmol/ml plasma.
To determine the fraction of protein-bound B12 in a given tissue, we measured the content of B12 before and after precipitation of free B12. Free B12 was precipitated employing B12-catching-beads; magnetic beads containing the B12 binding protein haptocorrin in its apo form. The beads were prepared as follows: Magnetic Dynabeads Pan Mouse IgG (Invitrogen catalogue no.11041) were coated with in-house monoclonal anti-haptocorrin as previously described [12] and incubated with recombinant human apo-haptocorrin (1.59 mg/ml - kind gift from Elaine Fisher, Zurich, Switzerland). After three washing cycles with wash buffer (5.5 mM Na2HPO4, 2 H2O (Merck, Damstadt, Germany), 1.2 mM NaH2PO4H2O (Merck, Damstadt, Germany), 139 mM NaCl (Merck, Damstadt, Germany), 1.5 mM human albumin (MP Biomedicals, USA), pH 7.4) the unsaturated B12 binding capacity was measured by adding increasing amounts of radiolabelled B12 (57Co-B12, Kem-En-Tec, Taastrup, Denmark) to a fixed volume of beads. The working solution was adjusted to have a B12 binding capacity of at least 160 fmol per 20 μl beads. We diluted each organ extract so to contain no more than 160 fmol B12 in a sample of 400 μl. Each sample was incubated with 20 μl B12-catching-beads for one hour at room temperature. B12 was measured prior to and after exposing the samples to B12-catching beads as described above, and the fraction of protein bound B12 was calculated by dividing B12 in supernatant by B12 present before adding of B12-catching-beads.
Statistical methods
A student's two-tailed unpaired t-test with Welch's correction was employed to explore differences between metformin treated and control rats. Prior to doing so we determined if data followed a Gaussian distribution employing the D'Agostino-Pearson omnibus test. For data found not to follow a Gaussian distribution we used the Mann-Whitney test for unpaired data. A two-tailed paired t-test was used to test differences between the start and the end of the experiment. We considered p < 0.05 as significant.
Results
We investigated the distribution of B12 in control rats aged 14 and 16 weeks, and studied the effect of metformin treatment administered either subcutaneously or by osmotic pumps. First we examined 16 weeks old rats treated with for six weeks with s.c. injections containing either saline or metformin. Key data on the rats are displayed in Table 1.
![]()
Table 1: Rats aged 16 weeks at sacrifice - Treatment by subcutanous injections
Total weight and weight of selected organs of 16 weeks old Wistar rats, treated for six weeks with daily subcutaneous injection of 150 mg/kg/day metformin (Metformin) or saline (Controls). All values are presented as median and [range]. Range indicates the lowest and the highest value observed. P-values < 0.05 were considered statistically significant.
View Table 1
Metformin treated rats gained less weight than did the control groups (p = 0.047). No difference in weight of the organs including kidneys, liver or hearts was observed.
The results of B12 measures from the 16 weeks old control rats are displayed in figure 1 and for both control and metformin treated rats in supplementary table also including B12 measures from rats aged 14 weeks. The kidney contained the highest amount of B12 both expressed per gram wet tissue (median 1350 pmol) and as total organ content (median 2440 pmol). Expressed per gram wet tissue the liver (78 pmol) contained around fifteen folds less than the kidney, but expressed per organ the difference was only 2.5 fold (1010 pmol in the liver).
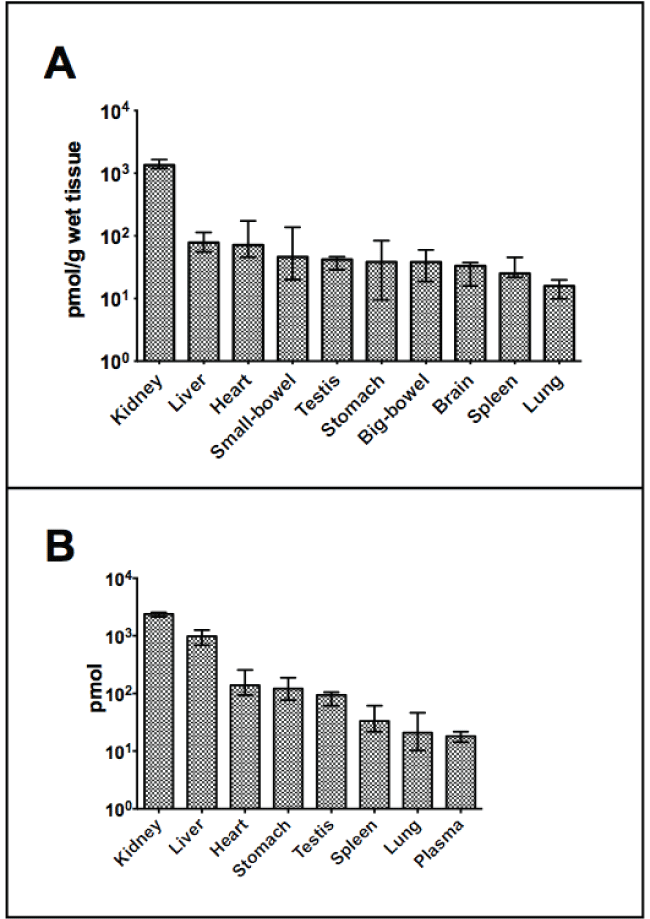
.
Figure 1: B12 in organs from 16 weeks old male Wistar rats (treated for six weeks with subcutaneous saline.
(A) pmol/g wet tissue with median (bar) and range (line). Range indicates the lowest and the highest value observed.
(B) Total amount per organ presented with median (bar) and range (line) in pmol. Y-axis is given as a logarithmic scale on both figures.
View Figure 1
We questioned to which extent tissue B12 occurred bound to proteins or not. For this purpose we selected tissue extracts from three randomly chosen control rats and measured B12 before and after precipitation of free B12 with B12-catching-beads. In the kidney around one third of B12 is present bound to proteins (mean fraction 0.32), while the major part of B12 occurred bound to proteins in all other organs examined, heart (0.86), liver (0.89), spleen and stomach (> 0.90).
In the second part of the study we used osmotic pumps to deliver either saline or metformin for four weeks to rats aged 14 weeks at sacrifice. This design was chosen to ensure a steady exposure to metformin. Three rats died due to postoperative complications, but the surviving rats showed a growth response comparable to those receiving subcutaneous treatment, with no difference observed between the metformin treated and the control rats. No difference was observed for the concentration of B12 in the liver and the kidney between controls and metformin treated rats (data not shown).
The 14 weeks old controls showed B12 values considerably lower than the 16 weeks old controls. The kidney contained (pmol/g [pmol/organ]) 65 [43] % and the liver 71 [63] % of the level present in the 16 weeks old rats.
Discussion
Here we report data on B12 distribution in B12-replete control rats and show no effect on the distribution of B12 following prolonged administration of metformin, neither subcutaneously nor delivered by intra-peritoneal osmotic pumps. Several studies in rats report the content of B12 in one or a few organs, and most often rat organs are investigated in states of either depletion or loading of B12. We find the kidney to contain more than fifteen fold more B12 per gram wet tissue than any of the other organs explored, which is in accord with previous investigations [4]. Interestingly we report the heart and the liver to contain a comparable amount of B12 expressed per gram of wet weight. In addition we find that rats accumulate more than 1.5 nmol of B12 between the age of 14 and 16 weeks.
In the cell B12 is bound to proteins in order to be converted into the coenzyme forms of the vitamin, methylcobalamin and 5'-deoxyadenosyl-cobalamin, and eventually to the two B12 dependent enzymes, methionine synthase and methylmalonyl- CoA mutase [13]. Thus the general concept is that most intracellular B12 is protein bound. We found this to be the case for most organs studied, where we observed the fraction of protein bound B12 to be above 85%. One exception was the kidney where less than one third of the B12 was present bound to proteins. The physiological role of the kidney in relation to B12 trafficking partly remains to be elucidated. Previous studies [3,4,14] present data which supports the kidney as a storage place for B12 in rodents. Studies in rats have shown that in the kidney B12 is contained within the lysosomes in the proximal tubular cells, and thus most likely present unbound to proteins. We calculated the total amount of B12 stored in the kidney to be 2440 pmol (median) and thus far exceeding the total amount of B12 present in the liver (median 1010 pmol). However, the fraction of protein-bound B12 was higher in the liver than in the kidney.
We have previously reported metformin treatment to result in a decline in plasma B12 not mirrored in the B12 content of the tissues in a rat model [1]. In our present study we could not reproduce the decline in plasma B12 but our data supports that metformin does not decrease the tissue levels of B12 in the rat.
Currently it remains unsettled whether metformin treatment induces B12 deficiency. Multiple population based studies have reported a B12 decline in serum upon treatment with metformin [6,15]. This has been attributed to malabsorption of B12 induced by metformin [7,16]. A recent meta-analysis of 22 clinical studies found a significantly increased risk of low levels of serum B12 associated with metformin treatment [10]. However, due to an inconsistent use of metabolic biomarkers in the studies included, the meta-analysis could not investigate the effect of metformin on other biomarkers of B12 metabolism (holotranscobalamin (holoTC), methylmalonic acid (MMA), and homocysteine), biomarkers that have been proposed more sensitive in detecting B12 deficiency than serum B12 [17,18]. Two recent clinical studies [9,19] included functional biomarkers (holoTC, MMA) in patients treated with metformin, along with serum B12. Despite significantly lower levels of total B12 in serum, these patients did not exhibit signs of B12 deficiency, based on measures of holoTC and MMA. On the contrary, one of the studies [9] found, that patients treated with metformin, exhibit superior intracellular metabolism, despite low values of B12 in serum. The results from our study are in accord with the recent clinical data, showing no effect of metformin on the B12 in serum or organs.
In conclusion we investigated B12 distribution in B12-replete control rats and in relation to treatment with metformin. Our results support recent findings suggesting that metformin does not influence neither the concentration nor the distribution of B12. In addition our study provides data on B12 in an extended number of organs from B12-replete rats.
Acknowledgements
The study was made possible through a research year scholarship awarded by MEMBRANES research centre at Aarhus University. In addition we would like to thank Jette Fisker and Inger Marie Jensen for technical assistance and advice.
Ethical Statement on the Use of Animals
The animal experiments and procedures described in this study was approved and carried out in accordance with the standards of The Animal Ethics Committee in Denmark.
References
-
Greibe E, Miller JW, Foutouhi SH, Green R, Nexo E (2013) Metformin increases liver accumulation of vitamin B12 - an experimental study in rats. Biochimie 95: 1062-1065.
-
Dryden LP, Hartman AM (1966) Relative concentration of vitamin B12 in the organs of the male rat as affected by its intake of the vitamin. J Nutr 90: 382-386.
-
Newmark P, Newman GE, O'Brien JR (1970) Vitamin B12 in the rat kidney. Evidence for an association with lysosomes. Arch Biochem Biophys 141: 121-130.
-
Birn H, Nexo E, Christensen EI, Nielsen R (2003) Diversity in rat tissue accumulation of vitamin B12 supports a distinct role for the kidney in vitamin B12 homeostasis. Nephrol Dial Transplant 18: 1095-1100.
-
de Jager J, Kooy A, Lehert P, Wulffelé MG, van der Kolk J, et al. (2010) Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 340: c2181.
-
Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP Jr (2012) Association of biochemical B12 Deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care 35: 327-333.
-
Tomkin GH, Hadden DR, Weaver JA, Montgomery DA (1971) Vitamin-B12 status of patients on long-term metformin therapy. Br Med J 2: 685-687.
-
Nielsen MJ, Rasmussen MR, Andersen CBF, Nexo E, Moestrup SK (2012) Vitamin B12 transport from food to the body's cells-a sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol 9: 345-354.
-
Obeid R, Jung J, Falk J, Herrmann W, Geisel J, et al. (2013) Serum vitamin B12 not reflecting vitamin B12 status in patients with type 2 diabetes. Biochimie 95: 1056-1061.
-
Niafar M, Hai F, Porhomayon J, Nader ND (2015) The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med 10: 93-102.
-
Lee HB, Blaufox MD (1985) Blood volume in the rat. J Nucl Med 26: 72-76.
-
Hardlei TF, Nexo E (2009) A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. Clin Chem 55: 1002-1010.
-
Banerjee R (2006) B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol 1: 149-159.
-
Birn H (2006) The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am J Physiol Renal Physiol 291: F22-36.
-
DeFronzo RA, Goodman AM (1995) Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 333: 541-549.
-
Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V (2000) Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 23: 1227-1231.
-
Nexo E, Hoffmann-Lucke E (2011) Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr 94: 359S-365S.
-
Obeid R, Herrmann W (2007) Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid. Clin Chem Lab Med 45: 1746-1750.
-
Greibe E, Trolle B, Bor MV, Lauszus FF, Nexo E (2013) Metformin lowers serum cobalamin without changing other markers of cobalamin status: a study on women with polycystic ovary syndrome. Nutrients 5: 2475-2482.





