Journal of Infectious Diseases and Epidemiology
Resistance Profile of Mycobacteria Isolated from Patients Undergoing Retreatment in Senegal
Mouhamadou Lamine Dia1*, Sow AI1, Cisse MF2, Gueye Pal3, Ba F3, Cisse NN3, Balde O3, Diouf B3 and Sarr M3
1Bacteriology-Virology Laboratory, Fann University Hospital Center, Dakar, Senegal
2Bacteriology-Virology Laboratory, Albert Royer Children's Hospital, Dakar, Senegal
3National Program Against Tuberculosis (PNT), Senegal
*Corresponding author:
Mouhamadou Lamine Dia, Bacteriology-Virology Laboratory, Fann University Hospital Center, BP 16222 Fann, Dakar, Senegal, Tel: 00-221-77-657-56-34, E-mail: laminedia2004@yahoo.fr
J Infect Dis Epidemiol, JIDE-2-012, (Volume 2, Issue 1), Short Note; ISSN: 2474-3658
Received: December 17, 2015 | Accepted: March 29, 2016 | Published: April 01, 2016
Citation: Dia ML, Sow AI, Cisse MF, Pal G, Ba F, et al. (2016) Resistance Profile of Mycobacteria Isolated from Patients Undergoing Retreatment in Senegal. J Infect Dis Epidemiol 2:012. 10.23937/2474-3658/1510012
Copyright: © 2016 Dia ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The aim of our study was to determine the resistance profile of mycobacteria isolated from patients who had treatment failure or relapse to first line anti-tuberculosis medication in Senegal.
Design: It involved 110 strains isolated in the National Program against Tuberculosis (PNT) laboratory between 2011 and 2012. These strains come from patients in treatment failure or relapse.
Results: They were from patients aged 9-73 years with a mean age of 36.605 years with a sex ratio (M/F) of 2.85. Antimicrobial susceptibility was carried out on 71 strains, 28 (39.43%) of which were from patients in treatment failure and 43 (60.56%) in relapsed patients. Among all patients, RMP presented the lowest resistance rates (43.66%). The highest resistance rates were noted in patients with treatment failure. In total, 31 (43.66%) MDR strains were found, including 21 patients in treatment failure (75%) and 10 relapsed patients (23.25%).
Conclusion: In Senegal the rate of anti-tuberculosis medication resistance among retreatment patients is high. Effective control measures and the availability of laboratory tests will allow better control of MDR-TB.
Keywords
M. tuberculosis, MDR-TB, PNT, Senegal
Introduction
Resistance to anti-tuberculosis medication is a major public health problem worldwide and Senegal is not spared. Indeed, the WHO reported 13,186 cases of tuberculosis in Senegal (including new cases and relapses) for 2013, 2.1% of which were multidrug-resistant tuberculosis (MDR-TB) among new cases and 17% in retreatment patients [1]. Elsewhere in the world, the WHO estimates the number of people infected with Mycobacterium tuberculosis (M. tuberculosis) at around 2 billion and the number of cases of MDR-TB at about 0.5 million, 60% of whom are concentrated in South Africa, Brazil, China, Russia and India ("BRICS" countries) [1]. Drug resistance to tuberculosis medication is therefore a major issue that jeopardizes the global fight against the disease and the success of DOTS, the strategy recommended by the WHO to detect and cure tuberculosis [2]. In Africa, few laboratories are able to demonstrate this resistance, which results in the under detection of this drug resistance. The aim of the study we carried out was to determine the resistance profile of mycobacteria isolated from patients in treatment failure or relapse to first line anti-tuberculosis medication in Senegal.
Materials and Methods
The study covered the period from January 2011 to December 2012, which is a total of two (2) years. It focused on clinically isolated strains in the national reference laboratory for mycobacteria as part of the National Program against Tuberculosis (PNT), Senegal.
These strains come from patients in treatment failure or relapse, which are defined as follows [2]:
- Treatment failure: a tuberculosis patient who continues to show positive results (by smear or cultivation) after five months or more of treatment with the combination of Rifampicin (RMP), Isoniazid (INH) Ethambutol (EMB) and Pyrazinamide (PZ) (RHEZ) for 2 months, then INH and RMP (RH) for at least three months.
- Relapse: patient who received a complete anti-tuberculosis treatment, i.e. two months of RHEZ, then 4 months of RH, who was declared cured at the end of treatment and again becomes bacilliferous.
These patients were from Dakar and other regions of Senegal. The clinical data were collected from the analysis reports accompanying the samples and included the following information: surname, first name, age, sex, clinical diagnosis, tuberculosis treatment history and referring structure. HIV status was not reported.
The samples were tested by conventional methods (microscopy, culture, biochemical identification and antibiogram).
Microscopic examination was carried out after Ziehl-Neelsen staining for the detection of acid-fast bacilli (AFB); bacteria were cultured on a simple Lowenstein-Jensen media or with pyruvate; the time to onset of colonies, the Niacin test, the investigation of a thermo labile catalase (at 22°C and 70°C) and the the detection of a nitrate reductase were used to identify strains.
Antibiotic resistance testing used the proportions method [3]. The samples were decontaminated with 4% NaOH and then centrifuged. The pellet was inoculated on a simple Lowenstein-Jensen (LJ), LJ supplemented with pyruvate and on LJ containing hydrazide thiophene-2-carboxylic acid (TCH) 5 mg/l. The antibiotics tested were: EMB 2 mg/l, SM 4 mg/l, INH 2 mg/l and the PMR 40 mg/l. A strain is declared resistant to a drug if its growth in the medium containing this TB is > 1% compared to the control [3].
The external quality control of bacteriological tests was carried out in the reference center for mycobacteria of the Institute of Tropical Medicine (ITM) in Antwerp, Belgium.
One sample was considered per patient. Data were entered and analyzed with EPI-INFO software, version 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Results
Our work focused on 110 M. tuberculosis strains. They come from the same number of patients (110), aged 9-73 years with an average age of 36.05 years and a sex ratio (M/F) of 2.85. These patients come from the regions of Dakar (88.99%), Thies (3.67%), Louga (4.59%), Diourbel (1.83%), Kaolack (0.92%). Of these, 41 (37.27%) had treatment failure and 69 (62.73%) relapsed.
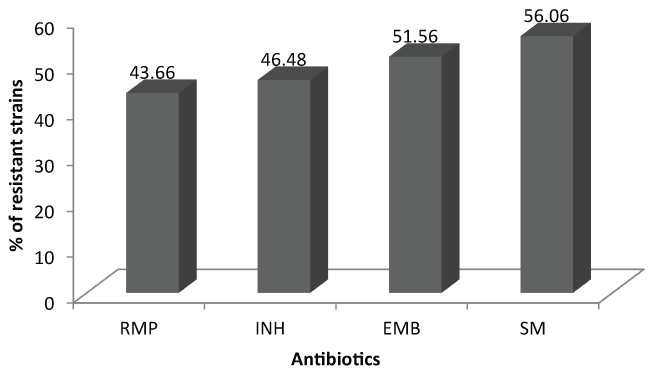
Antibiotic resistance testing was carried out on 71 strains, 28 of which (39.43%) came from patients who had therapeutic failure and 43 (60.56%) from relapsed patients. The number of tested strains is a little different about each antibiotic depending on the availability of drugs. Out of all patients, RMP presented the lowest resistance rates (43.66%) (Figure 1). High resistance rates to the different anti-tuberculosis drugs tested were found, with the highest resistance rates found in patients with treatment failure (Table I). In total 31 (43.66%) MDR strains were detected, including 21 in patients who had treatment failure (75%) and 10 relapsed patients (23.25%).
.
Figure 1: Global rate of resistance to different anti-tuberculosis medication tested.
View Figure 1
![]()
Table 1: Rate of resistance to anti-tuberculosis medication in patients with therapeutic failure or relapse.
View Table 1
Discussion
Our strains were isolated from patients in treatment failure or relapse. The strategy adopted in Senegal for the detection of cases of MDR-TB is the systematic cultivation for retreatment patients (failure, relapse, repeating treatment), tuberculosis contacts for MDR-TB, PLWHA and health personnel with tuberculosis [4]. Currently, the PNT has equipped five regions of the country with the Xpert MTB/RIF, which allows them to make a first screening, also allowing decentralization of the diagnosis of MDR-TB [4].
Our patients mainly come from the capital Dakar, but also from five other regions, so that our sample, although unevenly distributed, covers much of the country. The small number of samples from other regions is due to the constraints of transport to the PNT reference laboratory. They are predominantly made up of young adult males as reported in other studies, including African [5]. The mean age of 36.05 years found in our study is also comparable to data in the literature, especially African [5,6].
Overall, the high resistance rates that we obtained are related to the profile of the selected patients who are in treatment failure or relapse, therefore strongly suspected to have infection with resistant strains. Among the anti-tuberculosis drugs tested in all types of patient, RMP presented the lowest resistance rates (43.66%) while the highest rate was noted with SM (56.06%) with an overall MDR rate of 43.66%. HIV status was not determined in patients as the analysis of the literature shows that the best factor associated with the rate of MDR-TB for a country is the failure rate to retreatment while the incidence of tuberculosis or co-infection with HIV in Africa do not appear to be significantly associated with multidrug resistance [7].
We found the highest resistance rates in treatment failure patients with rates of 75%, 78.57%, 78.26% and 83.33% for RMP, INH, to EMB and SM, respectively. SM and EMB therefore presented the highest resistance rates and RMP the lowest rate in patients with treatment failure. These rates are higher than those found in Burkina Faso in previously treated patients (resistance rate of 51.6% to RMP, 66.7% to INH, 50.5% to EMB and 44.1% to SM) [8].
The overall rate of MDR strains that we found (43.66%) is comparable to that found in the Central African Republic (40% of MDR strains in patients with relapse or treatment failure) [9]. It is, however, below that found in Burkina Faso (50.5%) [8] and in the Ivory Coast (79%) [9]. This percentage of MDR strains was higher in patients with treatment failure with a rate of 75% versus 23.25% in patients with relapse. The WHO 2013 report on Senegal reported 2.1% of MDR-TB among new patients and 17% in patients treated in Senegal [1]. Our numbers are logically higher than those of the WHO report [1] linked to the profile of our patients who are in treatment failure or relapse, therefore strongly suspected of anti-tuberculosis drug resistance.
In fact, we have found two strains resistant to INH, but sensitive to RMP (INH-R/RIF-S strains). Indeed, RMP is often the last anti-tuberculosis to be affected by the resistance and the disease associated with INH defines MDR strains [10]. However, there are strains that are sensitive to INH but resistant to RMP, as other authors have found [10].
Conclusion
In Senegal, anti-tuberculosis drug resistance rates are very high among retreatment patients with a high percentage of MDR strains in patients with treatment failure. This multidrug resistance compromises the effectiveness of tuberculosis treatment with a high risk of spread of these MDR strains in the general population. Hence the importance of strengthening the fight against tuberculosis in general, in particular against MDR-TB. This necessarily involves an increase in the technical platform of laboratories for early detection of multidrug resistance. The initiative of the PNT aiming to equip all regions of Senegal with an Xpert MTB/RIF apparatus will ultimately enable earlier detection and better treatment of MDR-TB.
Acknowledgements
We would like to thank all the staff of the National Program against Tuberculosis (PNT) in Senegal, especially the laboratory staff and the hospital staff where the samples originate.
Ethical Statement
This retrospective study included data collected during routine diagnosis and treatment, so it did not require ethical approval.
Authors' contributions
DIA ML, GUEYE PAL, BA F, CISSE NN, BALDE O and DIOUF B participated in the samples testing by conventional methods (microscopy, culture, biochemical identification and antibiogram). SARR M, SOW AI and CISSE MF contributed to the writing and review of this article. All authors contributed to the writing of the manuscript, and all authors read and approved the final manuscript.
References
-
(2015) World Health Organization. TB country file: Senegal.
-
(2013) WHO. Definitions et cadre de notification pour la tuberculose - Revision.
-
Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, et al. (1963) Mycobacteria: Laboratory Methods for Testing Drug Sensitivity and Resistance. Bull Wld Hlth Org 29: 565-578.
-
(2013) National Antituberculosis Program (PNT), Senegal, Annual Report.
-
Mbatchou Ngahane BH, Diatta A, Toure NO, Dia Kane Y, Ba Diop S, et al. (2008) Clinical, biological and radiological spectrum of newly diagnosed pulmonary tuberculosis. Rev Mal Respir 25: 22-26
-
Diop SA, Fortes Deguenonvo L, Manga NM, Dia NM, Ka D, et al. (2014) Epidemiological, clinical and evolutionary aspects of tuberculosis retreatment patients. RMIM 3.
-
Abdelhadi O, Ndokaïn J, Moussa Ali M, Friocourt V, Mortier E, et al. (2012) Drug resistance testing of Mycobacterium tuberculosis isolates from sputum in Chad. Bull Soc Pathol Exot.; 105: 16-22.
-
Sangare L, Diande S, Badoum G, Dingtoumda B, Traore AS (2010) Anti-tuberculosis drug resistance in new and previously treated pulmonary tuberculosis cases in Burkina Faso. Int J Tuberc Lung Dis 14: 1424-1429.
-
Kouassi B, Horo K, N'douba KA, Koffi N, Ngom A, et al. (2004) Epidemiological, clinical and biological profile of pulmonary tuberculosis in situation of failure or relapse in Abidjan. Bull Soc Pathol Exot 97: 336-337.
-
Kurbatova EV, Cavanaugh JS, Shah NS, Wright A, Kim H, et al. (2012) Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other antituberculosis drugs. Int J Tuberc Lung Dis 16: 355-357.





