International Journal of Transplantation Research and Medicine
Pharmacological Conditioning of Brain Dead Donor Hearts with Erythropoietin and Glyceryl Trinitrate: Clinical Experience
Gayathri Kumarasinghe1,2,4*, Arjun Iyer1,3,4, Mark Hicks4,5, Alasdair Watson1,3,4, Hong Chew1,3,4, Ling Gao4, Jeanette Villanueva4, Andrew Jabbour1,2,4, Eugene Kotlyar1,2, Christopher S Hayward1,2,4, Anne M Keogh1,2,4, Emily Granger1,3, Paul C Jansz1,3,4, Kumud K Dhital1,3,4, Peter S Macdonald1,2,4 and Phillip Spratt1,3,4
1Heart & Lung Transplant Unit, St. Vincent's Hospital, Darlinghurst, Australia
2Department of Cardiology, St. Vincent's Hospital, Darlinghurst, Australia
3Department of Cardiothoracic Surgery, St. Vincent's Hospital, Darlinghurst, Australia
4The Victor Chang Cardiac Research Institute, Sydney, Australia
5Department of Clinical Pharmacology, St. Vincent's Hospital, Darlinghurst, Australia
*Corresponding author:
Gayathri Kumarasinghe, Heart & Lung Transplant Unit, St. Vincent's Hospital, 390 Victoria Street, Darlinghurst, NSW 2010, Australia, E-mail: g.kumarasinghe@victorchang.edu.au
Int J Transplant Res Med, IJTRM-2-018, (Volume 2, Issue 1), Review Article
Received: January 18, 2016: Accepted: March 30, 2016: Published: April 01, 2016
Citation: Kumarasinghe G, Iyer A, Hicks M, Watson A, Chew H, et al. (2016) Pharmacological Conditioning of Brain Dead Donor Hearts with Erythropoietin and
Glyceryl Trinitrate: Clinical Experience. Int J Transplant Res Med 2:018
Copyright: © 2016 Kumarasinghe G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:With the increasing success of heart transplantation, older and higher-risk donors and recipients are being accepted for transplantation. The risk of primary graft dysfunction (PGD) is thus increased. We investigated a 'pharmacological conditioning' strategy, where Celsior preservation solution supplemented with glyceryl trinitrate (GTN) and erythropoietin (EPO) was used for cardioplegia and hypothermic storage, and determined graft recovery and patient survival after cardiac transplantation.
Methods:Donor hearts retrieved between August 2010 and November 2013 were arrested and stored with supplemented Celsior (n = 61). Historical comparisons were made with hearts stored in Celsior (April 2005 to July 2010; n = 104) and modified St. Thomas' solution (STS, January 2000 to March 2005; n = 100). Donor, recipient and procedural risk factors for PGD were determined, and post-transplant use of mechanical circulatory support (MCS), length of stay (LOS) and 12-month survival compared between groups.
Results:Fewer hearts stored in STS came from donors aged > 50 years (p < 0.01). Recipients of hearts stored in supplemented Celsior had increased use of MCS pre-transplant (36%, p < 0.0001), and increased cross clamp times during implantation (111 min, p < 0.0001). Use of MCS post-transplant was 32.0%, 31.7% and 24.6% in STS, Celsior and supplemented Celsior groups respectively. There were no differences in LOS. Survival at 1-month was 92%, 95% and 98% and at 12-months 86%, 89% and 90% respectively.
Conclusion:Despite an increased donor and recipient risk profile, supplemented Celsior was associated with excellent operative and 12-month survival. There was a trend towards decreased need for post-transplant MCS, however the incidence of PGD remains high.
Keywords
Primary graft dysfunction (PGD), Donor heart preservation, Pharmacological conditioning, Ischemia-reperfusion injury (IRI), Hypothermic organ preservation
Introduction
The ongoing success and progress in heart transplantation has led to the acceptance of higher risk candidates for transplantation. These include patients of older age, with comorbidities, prior malignancies, redo sternotomies, and candidates on mechanical circulatory support (MCS) [1,2]. The rising demand for transplantation has also led to liberalization of donor acceptance criteria, with a significant increase in the use of extended criteria or 'marginal' donor hearts, in particular from older donors [1].
Transplanting higher risk recipients and using marginal donor organs increases the risk of post-transplant complications such as primary graft dysfunction (PGD) [3-7]. Particular emphasis has been placed on the additive effect of combining older donor age and prolonged ischemic time on the risk of PGD [1,8,9]. Certainly, longer ischemic times are encountered in the current era of transplantation, due to use of non-local donors and longer procedural times associated with technically complex recipients [1].
International Society for Heart and Lung Transplantation (ISHLT) registry data reveal that 39% of deaths in the first 30 days post-transplantation are due to PGD, with a further 18% attributed to secondary causes such as multi-organ failure [1,3]. A multi-center survey of autopsy results identified ischemia-reperfusion injury (IRI) as the main histopathological finding in 48% of deaths attributed to PGD [3]. Other findings such as myocyte necrosis (28%), multifocal edema and/or hemorrhage (14%) and freeze injury (7%) may also be attributed to injury incurred following prolonged hypothermic storage. Improving donor heart preservation is therefore of utmost importance, with organ retrieval a potential opportunity for therapeutic intervention.
Research in our institution has focused on applying myocardial 'conditioning' strategies to minimize IRI of the donor heart. The addition of the nitric oxide donor, glyceryl trinitrate (GTN), the glycoprotein hormone erythropoietin (EPO), and sodium-hydrogen exchange (NHE)-inhibitors to cardioplegia and hypothermic storage has demonstrated significantly improved post-storage recovery of cardiac output, myocardial contractility and reduced biochemical and histological markers of IRI in several preclinical studies [10-19]. The mechanisms of action of pharmacological conditioning are through activation of pro-survival signaling cascades mediated by ERK1/2, Akt of the reperfusion injury salvage kinase (RISK) pathway [20,21], STAT 3 of the survivor activating factor enhancement (SAFE) pathway [22,23], and cytoprotective effects of nitric oxide [24,25], that prevent oxidative stress, cellular edema, intracellular calcium overload and preserve mitochondrial integrity to prevent cellular apoptosis and necrosis due to IRI [26-32].
Given the success of these measures in multiple small and large animal studies including models that incorporate donor brain death, we aimed to assess the efficacy of pharmacological conditioning in reducing IRI in clinical cardiac transplantation. Two clinically available pharmacological conditioning agents EPO and GTN were added to cardioplegia and hypothermic preservation of the donor heart, with the aim of reducing primary graft dysfunction and improving patient survival. We report initial findings from this clinical study.
Methods
The study was conducted at a quaternary center specializing in cardiac transplantation and advanced heart failure care. Transplants performed between August 2010 and November 2013 used Celsior preservation solution supplemented with GTN and EPO for cardioplegia and hypothermic storage (n = 61). Data were compared with historic controls where Celsior alone was used (April 2005 to June 2010; n = 104) or modified St. Thomas' solution (STS, January 2000 to March 2005; n = 100).
Celsior preservation solution is classified as an unapproved therapeutic good in Australia. Approval to use Celsior for donor heart preservation was granted to one of the authors (PSM) in April 2005 by the Therapeutic Goods Administration (TGA) under Section 41HC of the Therapeutics Goods Act 1989. The TGA approval was endorsed by the St Vincent's Hospital Human Research Ethics Committee (HREC). TGA approval and HREC endorsement were renewed annually following submission of progress reports to both bodies. All potential heart transplant recipients provided written informed consent to the use of Celsior preservation solution and for their clinical information to be entered prospectively into a dedicated transplant database.
Donor, recipient and procedural data were obtained from a transplant database and medical records. Detailed data were collected to determine donor, recipient and procedural risk factors for PGD as outlined by Kobashigawa et al. in the 2013 Consensus Conference on Primary Graft Dysfunction after Cardiac Transplantation [3]. Transplants performed with organ retrieval by non-regional centers were excluded due to the use of other cardioplegic and hypothermic storage solutions.
Extended criteria (marginal) donor hearts
Donor hearts were classified as 'marginal' (extended criteria) according to the definition stated in the Transplantation Society of Australia and New Zealand Consensus Statement on Eligibility Criteria and Allocation Protocols for Organ Transplantation from Deceased Donors, 2010. That is, donor age ≥ 50 years, ischemic time > 360 minutes, donor left ventricular ejection fraction (LVEF) < 50% or regional wall motion abnormalities, the use of high dose inotropes (noradrenaline > 0.2 μg/kg/min or equivalent) or donor hepatitis B, C or high risk behavior.
Cardioplegia and hypothermic preservation
Antegrade cardioplegia was delivered via the aortic root in all transplants. Hearts were stored in hypothermic conditions (2-3°) during transport, using the same preservation solution as used in cardioplegia. All retrievals were performed by the study institution's transplant team.
Composition of preservation solutions
Celsior® was obtained commercially (Genzyme, Naarden, The Netherlands). Modified St. Thomas' Solution (STS) was prepared in-house, with the composition outlined in table 1. Both solutions were 'extracellular'-based preservation solutions with hyperkalemia and hypothermia the modes of induction of cardiac arrest. In the study group, Celsior® was supplemented with glyceryl trinitrate (Hospira Australia, Pty, Ltd., Mulgrave, AU) at a concentration of 0.1 g/L, and erythropoietin-alpha (Eprex; Janssen-Cilag, North Ryde, AU) at 5000U/L. Glyceryl trinitrate and EPO were added to Celsior® at the recipient institution, just prior to cardioplegia and hypothermic storage. The optimal concentrations of pharmacological supplements were determined from preclinical studies [10,18,19].
![]()
Table 1: Composition of preservation solutions.
View Table 1
Post-transplant outcomes and survival
Immediate post-transplant outcomes were determined by analysis of the use of mechanical circulatory support (extra-corporeal membrane oxygenation [ECMO] or intra-aortic balloon pump [IABP]) within the first 24 hours of transplantation. The length of stay in the intensive care unit (ICU) and length of hospital admission were compared between groups. Data were obtained for survival for 1, 3 and 12-months post-transplantation, with survival outcomes for all recipients obtained for the total 12 month follow-up period.
Definition of primary graft dysfunction
Primary graft dysfunction was defined by the use of ECMO or IABP in the first 24 hours after completion of cardiac transplantation surgery. Utilization of ECMO to treat severe PGD was instituted in our institution from 2005 onwards. Prior to 2005, all cases of PGD were managed by IABP support. Due to the retrospective nature of data collection, the use of inotropes and nitric oxide were not included in the analysis to determine mild left ventricular or right ventricular dysfunction. Echocardiography was routinely performed in the first hours post transplantation, however data were not used due to the absence of standardized reporting and recording in medical records. Reports of the first endomyocardial biopsy (week 1 post-transplant) were analyzed to exclude severe rejection as a cause of allograft dysfunction.
Statistical analysis
Continuous variables are represented as mean ± standard deviation and categorical data as the number of events and percentage. Differences between groups for continuous variables were calculated using one-way ANOVA with Tukey's post-hoc multiple comparisons test. Categorical data were analyzed using the Chi-square test. Survival data were analyzed using the Kaplan-Meier method. A p value < 0.05 was considered significant. All statistical analyses were performed using Prism 6 software (GraphPad Software, Inc., CA).
Results
Donor, recipient and procedural features
Donor, procedural and recipient risk factors for PGD are outlined for each group in table 2. There was a significantly higher proportion of hearts from donors ≥ 50 years of age in the supplemented Celsior group (25%) and Celsior alone (23%) compared with STS (9%, p < 0.01). Donors with anoxic brain injury, cerebral edema and meningitis were higher in the supplemented Celsior group (28%, p < 0.01) compared with Celsior and STS groups (19% and 8.5% respectively).
![]()
Table 2: Donor, procedural and recipient risk factors for primary graft dysfunction.
View Table 2
A higher proportion of recipients were on mechanical circulatory support prior to transplantation in the supplemented Celsior group (36%) compared with Celsior (22%, p < 0.0001) and STS (6.0%, p < 0.0001 supplemented Celsior; p < 0.01 Celsior). Procedural cross-clamp times were higher in the supplemented Celsior group (111 ± 36 minutes, p < 0.0001) compared with Celsior (83 ± 24 minutes) and STS (82 ± 20 minutes).
Other trends observed were an increase in the mean donor age in supplemented Celsior (38 ± 13 years) and Celsior groups (37 ± 14 years), compared with STS (33 ± 13 years, p = ns), and a higher proportion of redo-sternotomies in the supplemented Celsior group (48%) compared with Celsior (35%) and STS groups (35% and 34% respectively, p = ns). The proportion of donor hearts with left ventricular dysfunction at retrieval was similar in the three groups: supplemented Celsior (13%), Celsior (11%) and STS (10%, p = 0.85). There were no differences in mean recipient age or the presence of pulmonary hypertension across groups. Between 33 to 43% of ischemic times were > 240 minutes, with no significant differences across groups.
Post-transplant outcomes
Primary graft dysfunction, as defined by the use of ECMO or IABP within the first 24 hours of transplantation, was present in 25% of supplemented Celsior, 32% of Celsior and 32% of STS transplants (p = ns, Table 3). Veno-arterial (VA) ECMO was used in all cases except one patient in the Celsior group in whom veno-pulmonary arterial (V-PA) ECMO and an IABP were used. In marginal hearts, PGD occurred in 29% of supplemented Celsior, 37% of Celsior and 39% of STS transplants (p = ns). Severe rejection was not identified as the reason for implementation of MCS in any of the transplants. There were no significant differences in the length of stay in ICU or hospital between groups (Table 4).
![]()
Table 3: Use of ECMO or IABP post-transplantation.
View Table 3
![]()
Table 4: Length of stay: All transplants.
View Table 4
Of note, recipients in the STS group only received IABP support post-transplantation, which reflects practices in that era of transplantation (Table 3). The use of ECMO increased and the use of IABP decreased in Celsior and supplemented Celsior groups, also reflecting practices at the time.
Survival
Survival at 1-month post-transplant was 92%, 95% and 98% in STS, Celsior and supplemented Celsior groups respectively (p = ns, Figure 1). Survival at 3-months was 89%, 91% and 93% (p = ns) and at 12-months was 86%, 89% and 90% respectively (p = ns).
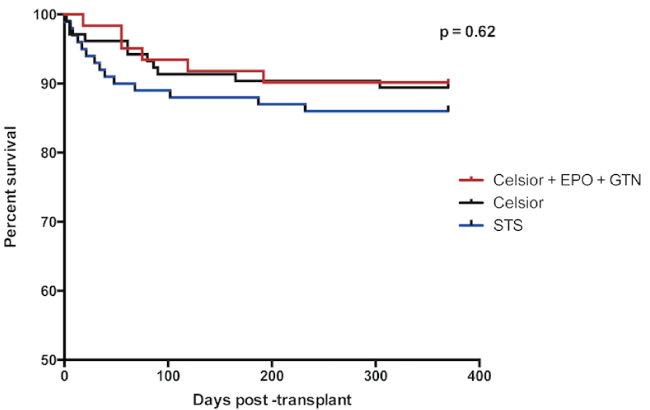
.
Figure 1: Survival (all hearts) at 370 days post-transplant.
Survival of patients with donor hearts stored in either St Thomas' Solution (STS), Celsior or supplemented Celsior (Celsior + EPO + GTN) are demonstrated for 370 days post-transplantation, with Kaplan-Meier analysis.
View Figure 1
Discussion
The introduction of brain dead donor heart conditioning with GTN and EPO at arrest, perfusion and storage at our institution has resulted in high patient survival despite increasing donor, procedural and recipient risk factors for PGD. The survival rate observed in our series compares favorably with ISHLT registry data, which reported post-transplant survival of 92% at 1-month, 90% at 3-months and 85% at 1-year in contemporaneous years of 2006 to 2011 [1,3]. To our knowledge, this is the first clinical study assessing the efficacy of pharmacological conditioning at improving preservation of donor hearts, and has demonstrated encouraging early results.
The need for MCS to treat PGD in our series is relatively high compared with other published series [33-35]. We believe this is explained in part by our relatively high utilization of hearts from marginal donors in an era when more than one third of recipients were supported on a VAD at the time of transplant. Indeed, in a recent US registry analysis, Fudim et al. [36] reported that implantation of marginal donors into VAD-supported patients was an independent risk factor for early graft failure and death. In the most recent era, 56% of donors had one or more marginal characteristic. Of particular note, 25% of donors in the most recent cohort were 50 years of age or older, and another 13% had evidence of left ventricular systolic dysfunction at the time of retrieval. In addition, due to large retrieval distances, the average ischemic time for heart transplants in our institution is one hour longer than the international average. The advent of peripheral ECMO as a relatively simple and safe option for short-term mechanical support [37] may have introduced two biases in the more recent era, firstly a greater preparedness on the part of surgeons to utilize marginal donors and secondly a move towards earlier utilization of ECMO to allow the heart more time to recover from the transplant process rather than attempt repeated weaning of a heart that is clearly struggling at the first attempt.
Future Directions
This first study on pharmacological conditioning of donor hearts in clinical cardiac transplantation has demonstrated safety and non-inferiority of this strategy of myocardial preservation. The translation of the pharmacological conditioning strategy was based on robust pre-clinical data which demonstrated significant improvements in the recovery of donor hearts in small and large animals [10-14,16-19,38]. These studies also demonstrated reduced biochemical markers of myocardial injury such as lactate dehydrogenase and troponin I, increased phosphorylation of key cell survival signaling enzymes ERK1/2, Akt, GSK-3β and reduction of markers of apoptosis such as cleaved caspase 3. A limitation in the translation of our pharmacological conditioning protocol from animal studies to man was that only two pharmacological agents, GTN and EPO are approved for clinical use. The third agent, a NHE-inhibitor, either zoniporide or cariporide, was not used due to discontinuation of clinical development of these agents due to safety concerns following the EXPEDITION trial [39]. In that trial, repeated bolus intravenous doses of cariporide for myocardial preservation in high-risk coronary artery bypass grafts demonstrated a higher incidence of fatal cerebrovascular accidents despite significantly improved myocardial protection. In the context of cardiac transplantation, NHE-inhibitors would only be used in cardioplegia and hypothermic storage of the donor heart and not administered systemically in the recipient, hence similar adverse effects are unlikely. Based on our previous finding of a synergistic interaction between zoniporide, EPO and GTN in preclinical studies, we believe that the addition of an NHE inhibitor would result in further improved myocardial preservation and reduced PGD if this 'triple supplementation' strategy is able to be implemented clinically.
We have also added EPO and GTN to STS in clinical heart transplants from donation after circulatory death (DCD) donors conducted at our institution [40]. These transplants utilized normothermic ex vivo perfusion (NEVP) for transport of the donor heart. The decision to flush the donor heart with STS rather than Celsior was based on the recommendation of the NEVP manufacturer. Recipients in the present study, and DCD transplants using pharmacological conditioning will be followed to determine longer-term effects of donor heart preservation, such as the incidence of cardiac allograft vasculopathy.
Limitations
There are a number of limitations to our study. Although all data were prospectively entered into a dedicated database, the analysis was conducted retrospectively. This was a single center study and historical controls were used as a basis for comparison. The use of historical controls however does highlight the dramatic changes that have occurred in both donor and recipient populations in little more than a decade. This study did not use hemodynamic criteria or echocardiographic data, hence all mild and some moderate cases of primary graft dysfunction were not captured in our data.
An era effect was demonstrated in practices of IABP and ECMO use. The use of ECMO, as opposed to ventricular assist device use for PGD has been associated with improved survival and is now considered a safer and more effective method of maintaining circulatory support in severe PGD [41]. Of patients treated with ECMO in our study, 80% one-year survival was demonstrated. Furthermore, while a reduction in PGD and an increase in survival were noted in the supplemented Celsior group, statistical significance was unable to be demonstrated due to relatively small patient numbers and hence the lack of statistical power. A prospective multi-center randomized controlled trial would be required to address these limitations and provide further support for our hypothesis that pharmacological conditioning improves donor heart preservation in the current era.
Conclusion
Early results of pharmacological conditioning demonstrate excellent survival rates in the immediate and early post-transplant period. The rate of primary graft dysfunction remains high and indicates an ongoing need for further improvements in donor heart preservation in an era of increasing donor and recipient risk profiles.
Funding Sources
This project was funded by the Heart & Lung Transplant Unit, St Vincent's Hospital, Sydney, NSW. Preclinical research was funded by the National Health and Medical Research Council of Australia. Scholarship funding was provided by the National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand.
Conflicts of Interest
The authors have no conflicts of interest to declare in relation to this manuscript.
Acknowledgements
The authors acknowledge the contributions of the Transplant Coordinators, Perfusionists and the Cardiothoracic Intensive Care Unit.
References
-
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, et al. (2014) The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report-2014; Focus Theme: Retransplantation. J Heart Lung Transplant 33: 996-1008.
-
Colvin-Adams M, Smithy JM, Heubner BM, Skeans MA, Edwards LB, et al. (2014) OPTN/SRTR 2012 Annual Data Report: heart. Am J Transplant 14 Suppl 1: 113-138.
-
Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F5, et al. (2014) Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 33: 327-340.
-
Chen JW, Chen YS, Chi NH, Huang SC, Yu HY, et al. (2014) Risk factors and prognosis of patients with primary graft failure after heart transplantation: an Asian center experience. Transplant Proc 46: 914-919.
-
Nilsson J, Ohlsson M, Stehlik J, Lund L, Andersson B, et al. (2015) Prediction of Primary Graft Dysfunction after Heart Transplantation. J Heart Lung Transplant 34: S35-S35.
-
Russo MJ, Iribarne A, Hong KN, Ramlawi B, Chen JM, et al. (2010) Factors associated with primary graft failure after heart transplantation. Transplantation 90: 444-450.
-
Segovia J, Cosío MD, Barceló JM, Bueno MG, Pavía PG, et al. (2011) RADIAL: a novel primary graft failure risk score in heart transplantation. J Heart Lung Transplant 30: 644-651.
-
Russo MJ, Chen JM, Sorabella RA, Martens TP, Garrido M, et al. (2007) The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg 133: 554-559.
-
Esmailian F, Patel J, Kittleson M, Kao T, Liou F, et al. (2015) Shorter Cold Ischemic Time in Older Donors Post-Heart Transplant Appears to Be Protective. J Heart Lung Transplant 34: S17-S17.
-
Cropper JR, Hicks M, Ryan JB, Macdonald PS (2003) Cardioprotection by cariporide after prolonged hypothermic storage of the isolated working rat heart. J Heart Lung Transplant 22: 929-936.
-
Gao L, Hicks M, MacDonald PS (2005) Improved preservation of the rat heart with celsior solution supplemented with cariporide plus glyceryl trinitrate. Am J Transplant 5: 1820-1826.
-
Gao L, Kwan JC, Macdonald PS, Yang L, Preiss T, et al. (2007) Improved poststorage cardiac function by poly (ADP-ribose) polymerase inhibition: role of phosphatidylinositol 3-kinase Akt pathway. Transplantation 84: 380-386.
-
Gao L, Tsun J, Sun L, Kwan J, Watson A, et al. (2011) Critical role of the STAT3 pathway in the cardioprotective efficacy of zoniporide in a model of myocardial preservation - the rat isolated working heart. Br J Pharmacol 162: 633-647.
-
Hing AJ, Watson A, Hicks M, Gao L, Faddy SC, et al. (2009) Combining cariporide with glyceryl trinitrate optimizes cardiac preservation during porcine heart transplantation. Am J Transplant 9: 2048-2056.
-
Iyer A, Gao L, Doyle A, Rao P, Jayewardene D, et al. (2014) Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant 14: 1744-1752.
-
Ryan JB, Hicks M, Cropper JR, Garlick SR, Kesteven SH, et al. (2003) Cariporide (HOE-642) improves cardiac allograft preservation in a porcine model of orthotopic heart transplantation. Transplantation 75: 625-631.
-
Ryan JB, Hicks M, Cropper JR, Garlick SR, Kesteven SH, et al. (2003) Sodium-hydrogen exchanger inhibition, pharmacologic ischemic preconditioning, or both for extended cardiac allograft preservation. Transplantation 76: 766-771.
-
Watson AJ, Gao L, Sun L, Tsun J, Doyle A, et al. (2013) Enhanced preservation of pig cardiac allografts by combining erythropoietin with glyceryl trinitrate and zoniporide. Am J Transplant 13: 1676-1687.
-
Watson AJ, Gao L, Sun L, Tsun J, Jabbour A, et al. (2013) Enhanced preservation of the rat heart after prolonged hypothermic ischemia with erythropoietin-supplemented Celsior solution. J Heart Lung Transplant 32: 633-640.
-
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61: 448-460.
-
Hausenloy DJ, Yellon DM (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12: 217-234.
-
Lacerda L, Somers S, Opie LH, Lecour S (2009) Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84: 201-208.
-
Lecour S (2009) Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol 47: 32-40.
-
Cohen MV, Yang XM, Downey JM (2006) Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70: 231-239.
-
Granger DN (1999) Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 6: 167-178.
-
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92-100.
-
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357: 1121-1135.
-
Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM (2002) Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 55: 534-543.
-
Hausenloy DJ, Yellon DM (2003) The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35: 339-341.
-
Hausenloy DJ, Yellon DM (2008) Preconditioning and postconditioning: new strategies for cardioprotection. Diabetes Obes Metab 10: 451-459.
-
Lim SY, Davidson SM, Hausenloy DJ, Yellon DM (2007) Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res 75: 530-535.
-
Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ (2015) The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 78: 23-34.
-
Marasco SF, Vale M, Pellegrino V, Preovolos A, Leet A, et al. (2010) Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann Thorac Surg 90: 1541-1546.
-
Lim JH, Hwang HY, Yeom SY, Cho HJ, Lee HY, et al. (2014) Percutaneous extracorporeal membrane oxygenation for graft dysfunction after heart transplantation. Korean J Thorac Cardiovasc Surg 47: 100-105.
-
Lima EB, da Cunha CR, Barzilai VS, Ulhoa MB, de Barros MR, et al. (2015) Experience of ECMO in primary graft dysfunction after orthotopic heart transplantation. Arq Bras Cardiol 105: 285-291.
-
Fudim M, Davis ME, Jenkins C, Brown CL, Wigger MA, et al. (2015) Marginal Donor Use in Patients Undergoing Heart Transplantation With Left Ventricular Assist Device Explantation. Ann Thorac Surg 100: 2117-2126.
-
Listijono DR, Watson A, Pye R, Keogh AM, Kotlyar E, et al. (2011) Usefulness of extracorporeal membrane oxygenation for early cardiac allograft dysfunction. J Heart Lung Transplant 30: 783-789.
-
Kwan JC, Gao L, Macdonald PS, Hicks M (2015) Cardio-protective signalling by glyceryl trinitrate and cariporide in a model of donor heart preservation. Heart Lung Circ 24: 306-318.
-
Mentzer RM Jr, Bartels C, Bolli R, Boyce S, Buckberg GD, et al. (2008) Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg 85: 1261-1270.
-
Dhital KK, Iyer A, Connellan M, Chew HC, Gao L, et al. (2015) Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 385: 2585-2591.
-
D'Alessandro C, Aubert S, Golmard JL, Praschker BL, Luyt CE, et al. (2010) Extra-corporeal membrane oxygenation temporary support for early graft failure after cardiac transplantation. Eur J Cardiothorac Surg 37: 343-349.





