International Journal of Respiratory and Pulmonary Medicine
Outcome Predictors of Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease: Role of Inflammatory Biomarkers
Hanaa Shafiek1*, Nashwa Abd-elwahab1, Manal Baddour2, Akram Degady3, Mohamed El-hoffy1 and Yehia Khalil1
1Chest Diseases Department, Faculty of Medicine, Alexandria University, Egypt
2Microbiology and Immunology Department, Faculty of Medicine, Alexandria University, Egypt
3Clinical and Chemical Pathology Department, Faculty of Medicine, Alexandria University, Egypt
*Corresponding author: Hanaa Shafiek, MD, PhD, Chest Diseases Department, Faculty of Medicine, Alexandria University, El-Khartoom Square, Alexandria 21526, Egypt, Tel: 002-01095907320, E-mail: whitecoat_med@yahoo.com
Int J Respir Pulm Med, IJRPM-3-047, (Volume 3, Issue 2), Original Research; ISSN: 2378-3516
Received: February 06, 2016 | Accepted: May 05, 2016 | Published: May 09, 2016
Citation: Shafiek H, Abd-elwahab N, Baddour M, Degady A, El-hoffy M, et al. (2016) Outcome Predictors of Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease: Role of Inflammatory Biomarkers. Int J Respir Pulm Med 3:047. 10.23937/2378-3516/1410047
Copyright: © 2016 Shafiek H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To assess the value of interleukin-6 (IL-6), interleukin-8 (IL-8) and C-reactive protein (CRP) in predicting the outcome of acute respiratory failure (ARF) on top of COPD.
Patients and methods: Serum samples were collected from 33 COPD patients presented with ARF for IL-6, IL-8, and CRP analysis on admission, after 72 hours and after 14 days. Sputum samples were taken for microbiological evaluation.
Results: During the study, 75.8% survived and 24.2% died. Serum IL-6 only on admission was significantly higher (p = 0.03) among the non-survivors [376.0 (interquartile range (IQR) = 26.3-511) pg/ml] vs. the survivors [8.2 (IQR = 0.1-17) pg/ml] as well as after 14 days (p = 0.04). Both the CRP and IL-8 were higher among the non-survivors without significant difference (p > 0.05). The IL-6 after 72 hours showed statistical significance (p = 0.03) in predicting the outcome being lower among those discharged on room air [0.1 (IQR = 0.1-0.3)pg/ml] compared to either the non-survivors or those discharged on new supplemental oxygen therapy or continuous positive airway pressure [1.6 (IQR = 0.1-187.6) and 7.2 (IQR = 0.1-41.3) pg/ml respectively]. IL-6 > 46.1 pg/ml on admission had the sensitivity of 71% and specificity of 84% for predicting in-hospital mortality (p = 0.042); but CRP > 2.3 mg/L had the best sensitivity (85.7%). Gram-negative bacteria- the commonest pathogen among non-survivors- was insignificantly associated to the outcome (p = 0.262).
Conclusions: High IL-6 is associated with in-hospital mortality in ARF on top of COPD. Both CRP and IL-6 when used together, they become good in-hospital mortality predictors.
Keywords
Acute respiratory failure, Interleukin-6, In-hospital mortality, C-reactive protein, Gram-negative bacteria
Introduction
Acute exacerbations of chronic obstructive pulmonary diseases (AECOPD) are an important problem for healthcare systems, because of the morbidity and mortality rates. These episodes are associated with accentuation of both airway and systemic inflammation [1] besides the worsening in airway function and respiratory symptoms in patients with chronic obstructive pulmonary diseases (COPD) [2] AECOPD can range from self-limited episodes to acute respiratory failure (ARF) requiring mechanical ventilation [3]. A range of potential factors have been studied both as risk factors of exacerbations resulting in hospitalization as well as predictors of mortality and other outcomes [4]. These factors include forced expiratory volume in one second (FEV1), partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide(PaCO2), oxygen saturation and resting oxygen uptake, low body mass index (BMI), current smoking status, older age, low serum albumin, the comorbidities, severity of illness and functional status [4].
Various markers were reported to be higher in blood during exacerbation compared with the baseline include C-reactive protein (CRP), interleukin 8 (IL-8), tumor necrosis factor- α (TNF-α), leptin, endothelin-1, eosinophil cationic protein, myeloperoxidase, fibrinogen, interleukin 6 (IL-6), α1-antitrypsin, and leukotriene E4, leukotriene B4 and copeptin [5]. The aim of the present study was to assess the value of some inflammatory biomarkers (IL-6, IL-8 and CRP) in predicting the outcome of ARF on top of COPD, to propose cutoff value of the studied biomarkers for predicting in-hospital mortality and assessing its sensitivity and specificity. Part of this study have been published [6].
Methods
Study design and population
A cross sectional prospective pilot study enrolled 33 patients with COPD diagnosis as defined by GOLD [7] presented with acute respiratory failure attending the respiratory intensive care unit (RICU) of the chest diseases department, Alexandria Main University Hospital, Alexandria, Egypt between June 2010 and August 2011. All the patients were followed up for 30 days. The study was approved by local Ethical Committee.
Characteristics of the patients
All patients at recruitment were in acute respiratory failure as defined by arterial blood gas (ABG) criteria (PaO2 < 60 mmHg, with or without PaCO2 > 45 mmHg/Ph < 7.35) during breathing room air [8] with the presence of acute respiratory distress. Further, all the patients were not admitted to the hospital in the last 30 days before recruitment. Any patient suffering from other confounding inflammatory diseases, such as malignancy, arthritis, connective tissue disorders or inflammatory bowel disease as well as all patients with other significant respiratory diseases including asthma, tuberculosis and primary bronchiectasis were excluded from the study.
All the patients on admission were subjected to thorough history taking (including age, gender, smoking history, exacerbations/year, and comorbidities). Baseline dyspnea was evaluated using "The Modified Medical Research Council (MMRC) dyspnea scale scoring" [9] full clinical examination including weight and height, laboratory investigations (mainly complete blood picture, serum albumin, serum electrolytes, creatinine, blood urea nitrogen), plain chest X-ray and ABG.
After initial evaluation, the patients were managed according to the international guidelines [10]. The patients were assigned to the standard drug protocol, supplemental oxygen therapy plus NIV as initial trial if not contraindicated and maintained as long as it is tolerated. The patients were monitored during the NIV for oxygen saturation, clinical state and hemodynamic state. ABG was further ordered if the patient did not improve or showing clinical evidence of deterioration. Failure of NIV was defined as termination of NIV trial and initiation of invasive mechanical ventilation (MV).
Microbiological evaluation
Sputum samples or bronchial wash -performed for selected cases- were obtained on admission from all patients for microbiological analysis using both ordinary cultures for common pathological bacteria and polymerase chain reaction (PCR) technique for atypical pathogens (Mycoplasma and Chlamydia). Details of the bacteriological detection were previously publication [6]. Briefly, an equal volume of the sputum was mixed with sterile saline and incubated at room temperature for 15 minutes with intermittent shaking for homogenization of sputum. For the bronchial wash, no dilution was done. Samples were inoculated on Blood agar and MacConkey's agarfor quantization of pathogens. Identification of the infecting pathogens was carried out according to standard microbiological procedures including Gram staining and biochemical reactions followed by antibiotic susceptibility testing. Polymerase chain reaction (PCR) was done on the collected sputum or bronchial lavage sample for detection of Mycoplasmapneumoniae and Chlamydia pneumoniae. DNA was extracted from all samples using the GeneJET™ Genomic DNA Purification Kit (Fermentas, Thermo Scientific) followed by amplification of DNA sequence which was carried out in a Techne Progene thermal cycler. Electrophoresis was carried out at 80 volts for 25 minutes in order to detect the amplified products. DNA fragments (280 bp for Mycoplasma pneumoniae and 474 bp for Chlamydia pneumoniae) were visualized by ethidium bromide staining against an ultraviolet transilluminator [11].
Serum samples and analysis of the inflammatory markers
Serum samples were obtained (on admission, after 72 hours and on the 14th day) and were preserved in -80°C till the end of the study period. The collected samples were analyzed for IL-6, IL-8 and CRP. The IL-6 and IL-8 were measured in serum samples by ELISAkit (AviBion human IL-6 and IL-8, code number IL06001 and IL08001 respectively, Finland) according to the manufacturers' directions. CRP was measured in serum samples by CRP-ultrasensitive (MICRO CRP/ ULTRA CRP, Vital Diagnostics, Italy). This kit utilizes quantitative turbidimetric latex technique.
Statistical analysis
Quantitative data are presented as means ± standard deviation (SD) while categorical variables as frequencies and percentages (%) unless otherwise stated. All statistical tests including Kruskal-Wallis test, Mann-Whitney test, Monte-Carlo test and Spearman rank correlations were employed as appropriate unless otherwise stated. Post hoc testing was used for multiple comparisons corrections. Receiver Operating Characteristic (ROC) curve analysis and area under the curve (AUC) were used to identify the best cut-off points of each of IL-6, CRP, and IL-8 and calculate its sensitivity and specificity in predicting in-hospital mortality. Cox regression hazard and Kaplan Meier analysis were applied to study different parameters dependency of survival. Both the hazard ratio and 95% confidence interval (CI) were shown. All applied statistical tests of significance were two-sided and p ≤ 0.05 was considered as statistically significant. Statistical analysis was carried out using Microsoft excel and Statistical Package for Social Sciences (SPSS version 11, Chicago, Il, USA).
Results
Patients' characteristics
Baseline clinical and physiological characteristics of the 33 patients are shown in table 1. The majority of our patients were severe to very severe COPD with average FEV1 % predicted of 43.7 ± 18.2%. Twenty-five patients (75.8%) survived and 8 patients (24.2%) died during the study; where 2 patients only of non-survivors (6%) were alive on the 14th day and died on days 17th and 20th of the study. Those who survived were discharged from the hospital either on room air [14 patients (43%)] or new supplemental oxygen therapy/ CPAP [11 patients (33%)]. The causes of mortality were either new insult i.e., hospital acquired infection and tension pneumothorax, the persistence of initial infection with progression to shock and a baseline of very severe COPD. The characteristics of the two groups (survivors and non-survivors) are shown in table 2.
![]()
Table 1: Demographic and clinical characteristics of recruited patients.
View Table 1
![]()
Table 2: Comparison between the survivors and non-survivors regarding different clinical, physiological, radiological and laboratory variables.
View Table 2
There was a statistically significant difference between the survivors and non-survivors regarding the BMI index (29.8 ± 7.8 versus 21.6 ± 4.2 kg/m2) and dyspnea grade (2.4 ± 0.9 versus 3.6 ± 0.5) (p = 0.008 and 0.001 respectively; table 2). In addition, there was a statistically significant difference between the chloride level of the survivors (96.6 ± 3.6 mmol/L) and that of the non-survivors (92 ± 6.6 mmol/L) (p = 0.034; table 2). However, there was no significant difference between the two groups regarding the age, exacerbation history, smoking status and index, ABG, failure of NIV trial, blood urea nitrogen, creatinine, the remaining electrolytes, serum albumin, the hematocrit and total white blood count as shown in table 2.
Regarding the plain chest X-ray taken on admission there was no statistically significant difference between the survivors and non-survivors concerning the presence of consolidative patches (p = 1.0); however, on the 14th day 25% of the non-survivors acquired new patch indicating hospital acquired infection which gave a statistically significant difference when compared to survivors (4% only had non-resolving patch) (p = 0.01; table 2).
Microbiologically
Causative microorganism was detected in 66% of the sputum samples. Gram-negative bacteria were detected in 9 sputum samples (31%). Atypical bacteria were identified in 8 patients (28%) in the form of Mycoplasma (25%) mainly; more details regarding microorganism cultured could be reviewed in our previously publication [6]. Gram-negative bacterial infection was the most frequent (66.7%) among the non-survivors. The atypical bacterial infection was the most frequent (36.4%) among the patients discharged on O2 therapy and/or CPAP, but undetected bacteria were more prevalent (41.7%) among those discharged on room air. However, there was no statistically significant association between the type of infection and the outcome of the patients on the 14th day (p = 0.262).
Inflammatory biomarkers
IL-6 showed a statistically significant difference between the survivors and non-survivors on admission 8.2 (interquartile range (IQR) = 0.1-17) vs. 376.0 (IQR = 26.3-511) pg/ml respectively; p = 0.03, table 3). Similarly, on the 14th day there was a statistically significant difference between the survivor group 3.1 (IQR = 0.1-23.2) vs. non-survivor group (2 patients) 76.3 (IQR = 68.5-84.1) pg/ml; p = 0.04, table 3).
![]()
Table 3: Comparison between levels of studied biomarkers (IL-6, IL-8 and CRP) at different stages of the study according to survival of patients$.
View Table 3
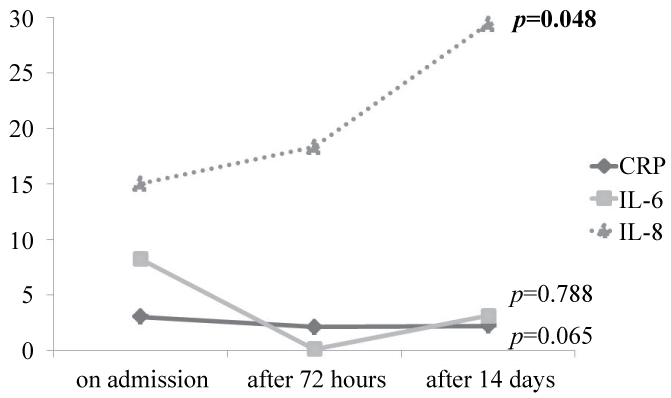
Further, IL-6 level after 72 hours has statistically significant difference in predicting the outcome on the 14th day (p = 0.03, table 4) as those discharged on room air had the lowest value (0.1 (IQR = 0.1-0.3) pg/ml) compared to either the non-survivors or those discharged on oxygen therapy or CPAP (1.6 (IQR = 0.1-187.6) and 7.2 (IQR = 0.1-41.3) pg/ml respectively). However, neither the CRP nor the IL-8 showed any significant difference when comparing the survivors and non-survivors or in predicting the outcome (p > 0.05; tables 3 and 4). Further, the inflammatory biomarkers did not show regression among the survivors over the study durationdespite the apparently initial regression in IL-6 values after 72 hours (figure 1).
.
Figure 1: The inflammatory biomarkers among the survivors at different stages (on admission, after 72 hours and after 14 days) of the study.
View Figure 1
![]()
Table 4: Comparison between the levels of studied inflammatory biomarkers on admission and after 72 hours according to the outcome on the 14th day$.
View Table 4
Correlations
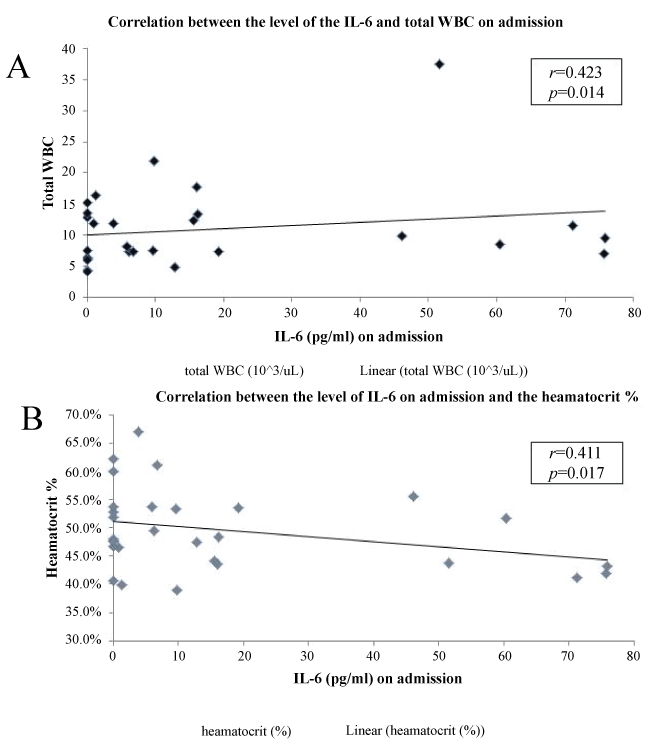
There was no statistically significant correlation between the studied inflammatory markers' level on admission and the following: BMI, dyspnea grade, the exacerbation history, the smoking status and the associated co-morbidities (p > 0.05). However, a statistically significant positive correlation was found between the IL-6 and the total white blood count and inverse correlation with the hematocrit value (r = 0.423, p = 0.014; r = -0.411, p = 0.017 respectively; figure 2). Moreover, there was a statistically significant positive correlation between CRP and IL-6 (r = 0.298, p = 0.005). In addition, there was a statistically significant positive correlation between the CRP and the smoking index (r = 0.514, p = 0.007).
.
Figure 2: Correlation between IL-6 level on admission and both white blood count (A) and heamatocrit (B).
View Figure 2
ROC and regression analysis
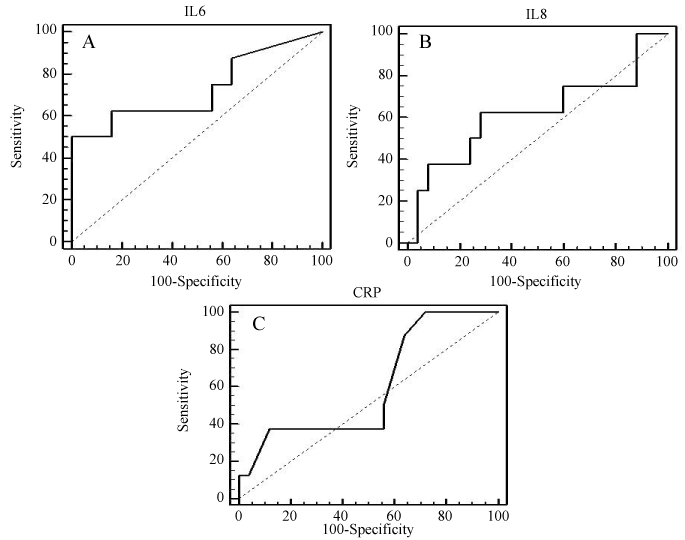
The cutoff point of IL-6 is > 46.1pg/ml which shows a sensitivity of 71% for predicting "in-hospital mortality" and a specificity of 83% (AUC= 0.77, p = 0.042; figure 3-A). The cutoff point of IL-8 is > 28.6pg/ml which has a lower sensitiviy (57%) and specificity (72%) (AUC = 0.583, p = 0.565; figure 3-B). Regarding CRP, the cutoff point is > 2.3 mg/L which has high sensitivity (85.7%) for predicting mortality but the lowest specificity (37.5%) (AUC = 0.563, p = 0.615; figure 3-C).
.
Figure 3: ROC analysis of studied biomarkers on admission regarding the in-hospital mortality; A. ROC curve for IL-6 (AUC = 0.77, p = 0.042); B. ROC curve for IL-8 (AUC = 0.583, p = 0.565); C. ROC curve for CRP (AUC = 0.563, p = 0.615).
View Figure 3
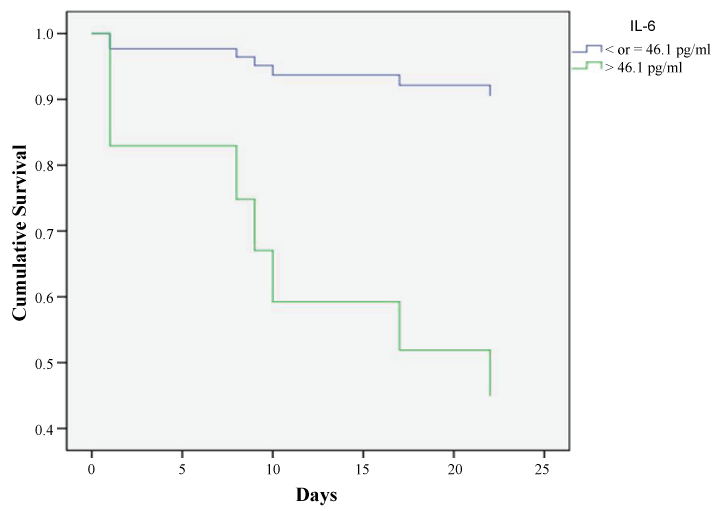
Moreover, Cox proportional hazards regression analysis showed that only IL-6 value on admission was strongly associated to the probability of in-hospital mortality with a hazard ratio of 2.5 (CI 95% = 1.2-5.01; p = 0.013) with 60% probability of survival at 14 days in case of value > 46.1 pg/ml (figure 4); while the value of the same biomarker at 14 days had a hazard ratio of 3.4 (CI 95% = 0.7- 16.3) but it did not reach statistical significance (p = 0.132). None of the other biomarkers or the ABG parameters showed significant hazard ratio in relation to in-hospital mortality.
Discussion
In the current study we showed accentuated systemic inflammation in non-survivors presented with severe AECOPD especially regarding IL-6. Further, IL-6 level on admission was the only biomarker with high specificity for predicting in-hospital mortality during AECOPD associated with ARF rather than CRP and IL-8 despite the high sensitivity of CRP.
Previous studies
Seneff et al. [12] and Ai-Ping et al. [13] showed that mortality was not related to baseline functional capacity, comorbidities including the presence of cor-pulmonale, ABG, previous hospitalization, or the use of invasive MV. Also, Aburto et al. [14] found no significant relationship between the admission ABG, serum albumin or hematocrit levels and the in-hospital mortality rate; and Potgieter et al. [15] found that the occurrence of nosocomial pneumonia is associated with an increased risk of fatalities. Additionally, Ong et al. [16] and McGhan et al. [17] showed in their studies that dyspnea score and weight loss respectively can predict the in-hospital mortality among admitted AECOPD patients being linked to increased systemic inflammation as expressed by elevated CRP, TNF-α or IL-6 [18,19]. Our results agreed with these observations. Further, the overall in-hospital mortality in our RICU was 24.2% which is within the range of previous studies [12,20].
Interpretation of the main results
We found that all the studied inflammatory biomarkers were higher among the non-survivors rather than the survivors with a statistically significant difference regarding IL-6; and that IL-6 value > 46.1 pg/ml on admission showed the highest specificity (83%) in predicting in-hospital mortality and was associated with reduced survival to 60% at 14 days. Interestingly, IL-6 value after 72 hours was significantly higher among the non-survivors and the survivor patients discharged on O2 therapy or CPAP and the least value was among those discharged on room air. These findings raise the potential of the strong prognostic value of IL-6 and could reflect severe underlying lung disease. This could be explained on the basis that activated epithelial cells and increased numbers of alveolar macrophages and other inflammatory cells in COPD may release IL-6 into the circulation [21] which in turn increased during AECOPD [1,22]. Various mechanisms demonstrated the role IL-6 in the pathogenesis of COPD. Firstly, IL-6 increases the number of lung CD4 cells, CD8 cells, B cells, neutrophils, and macrophages [23,24] consistent with the changes observed in human COPD pathology [25]. Secondly, overexpression of IL-6 leads to emphysema-like airspace enlargement, peribronchiolar collections of mononuclear cells, thickening of airway walls, subepithelial fibrosis, and airway hyperresponsiveness [23,26]. Lastly, lung injury is attenuated by the absence of IL-6 after exposing animals to ozone [27].
Furthermore, there was a statistically significant positive correlation between the IL-6 and total WBC which supports the concept of increased systemic inflammation associated with AECOPD [1,22,28]. Also, IL-6 inversely correlated with the haematocit in the present results. It has been proven that the increased levels of inflammatory cytokines lead to a shortened RBC survival, hence predispose to anemia [29]. Markoulaki et al. [30] found that during admission for AECOPD, hemoglobin levels are decreased and erythropoietin hormone levels are increased. This association is mainly related to increase IL-6 levels, indicating a possible erythropoietin resistance through the mechanism of increased systemic inflammatory process [30]. Additionally, IL-6 correlated positively with CRP; this is due to the fact that the CRP is primarily produced by hepatocytes in response to IL-6 stimulation [31].
We found that the chloride level was significantly lower among the non-survivors compared to the survivors. McMahon et al. [32] who analyzed the data of 51,789 critically ill patients found that hypochloremia proved to be an independent predictor of mortality of all-cause mortality among critically ill patients, even after adjustment for sodium, although the mechanisms for this association remained unclear. However, hypochloremia is caused by multiple factors including: a) extrarenal causes as inadequate NaCl intake, losses of gastrointestinal fluids (e.g. vomiting, nasogastric suction); b) renal causes (e.g. diuretic abuse); c) conditions associated with adrenal insufficiency (e.g. lack of endogenous or exogenous glucocorticoids or mineralocorticoids), d) dilutional causes as early stages of hyperglycemia, syndrome of inappropriate antidiuretic hormone, decreased effective circulatory blood volume as in edema states), e) acid-base abnormalities (e.g. compensated respiratory acidosis); all of these causes are commonly associated with the critically ill patients [33].
According to the current results, we observed that the inflammatory biomarkers did not regress significantly during recovery among the survivors especially for IL-8 (Figure 2). However, IL-6 showed insignificant regression after 72 hours which could be due to therapeutic agents as corticosteroids [34] and antibiotics that modulate the inflammatory cascade [35]. Some studies [36-38] previously reported insignificant improvement of the biomarkers during recovery from AECOPD up to 2 months [38] denoting persistent systemic inflammation post-exacerbation. Kersul et al. [39] also observed similarly continue high levels of inflammation after resolution of exacerbation episode especially regarding IL-8.This could be explained by the molecular mechanism that regulates the transcription of inflammatory genes in the cell nucleus which is basally reduced in COPD [40] does not increase during treatment of the exacerbation with glucocorticoids.
Finally, neither the CRP nor the IL-8 showed good specificity for predicting in-hospital mortality despite the highly detected sensitivity of CRP (87.5%). This could be explained by the fact that CRP by itself is not specific in case of AECOPD [41] and could be affected by many factors as inflammatory response elsewhere in the body, infection, antibiotic treatment and use of inhaled corticosteroids [42] as well as smoking index [43] that positively correlated with CRP in the current study.
Clinical implications
Our pilot study demonstrates the role ofIL-6 in severe AECOPD and its relation to fatal outcome among this group of patients. Accordingly, we suppose that incorporation of IL-6 in the early evaluation of AECOPD could help better identification of the severe episodes especially of being inexpensive and easy test. Secondly, directed therapy against IL-6 or its receptor could improve the outcome of AECOPD by decreasing the burden of systemic inflammation in COPD patients which could be the nidus for frequent AECOPD [44] especially if infection is not the cause. Directed therapy against inflammation has been raised during the last decade [45].
Limitations of the study
The present study has some limitations. Firstly, we studied only the systemic inflammation and we did not evaluation the extent of the airway inflammation during severe AECOPD. However, some studies previously showed that during the exacerbation of COPD, the systemic inflammation reflects in an acceptable way the airway inflammation [1]. Secondly, we did not evaluate the validity of IL-6 or the other studied biomarkers (IL-8 and CRP) as long-term prognostic factors for patients presented with severe AECOPD as our study was for short term.
Conclusion
High serum levels of IL-6, lower BMI, higher dyspnea grade, low serum chloride, newly developed consolidative patch in the plain chest X-ray on follow up were predictors of bad prognostic outcome and high fatality rate. Further, IL-6 (> 46.1 pg/ml on admission) could be considered as single biomarker with good sensitivity and specificity for predicting in-hospital mortality. Also, CRP showed high sensitivity in predicting in-hospital mortality despite being non-specific. Accordingly, both of CRP and IL-6 when used together, they become good mortality predictors.
Acknowledgments
The authors thank the participants for their willingness to contribute to medical research and the team work of our RICU for their support. All the authors are grateful to Prof. Mona Shawky, professor of epidemiology and statistics, for reviewing the statistics of the present work. H. Shafiek also would like to deeply thank Prof. Tamer Saed for his continuous support and encourage on life and research.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
-
Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA (2006) Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 71-78.
-
Celli BR, MacNee W. ATS/ERS Task Force (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. EurRespir J 23: 932-946.
-
Celli BR, Barnes PJ (2007) Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 29: 1224-1238.
-
Groenewegen KH, Schols AM, Wouters EF (2003) Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 124: 459-467.
-
Wouters EF, Groenewegen KH, Dentener MA, Vernooy JH (2007) Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc 4: 626-634.
-
Shafiek HA, Abd-Elwahab NH, Baddour MM, El-Hoffy MM, Degady AA, et al. (2012) Assessment of some inflammatory biomarkers as predictors of outcome of acute respiratory failure on top of chronic obstructive pulmonary disease and evaluation of the role of bacteria. ISRN Microbiol 2012: 240841.
-
(GOLD) NWGIfCOLD (2009) Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease.
-
Weiss SM, Hudson LD (1994) Outcome from respiratory failure. Crit Care Clin 10: 197-215.
-
Mahler DA, Wells CK (1988) Evaluation of clinical methods for rating dyspnea. Chest 93: 580-586.
-
British Thoracic Society Standards of Care Committee (2002) Non-invasive ventilation in acute respiratory failure. Thorax 57: 192-211.
-
Bedewy KML EM, Mahmoud MI (2001) Ventilator-associated pneumonia: serum TNF8 and determination of the role of some atypical bacterial pathogens by PCR. Egyptian Journal of Medical Microbiology 10: 27-42.
-
Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA (1995) Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 274: 1852-1857.
-
Ai-Ping C, Lee KH, Lim TK (2005) In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest 128: 518-524.
-
Aburto M, Esteban C, Moraza FJ, Aguirre U, Egurrola M, et al. (2011) COPD exacerbation: mortality prognosis factors in a respiratory care unit. Arch Bronconeumol 47: 79-84.
-
Potgieter PD, Hammond JM (1992) Etiology and diagnosis of pneumonia requiring ICU admission. Chest 101: 199-203.
-
Ong KC, Earnest A, Lu SJ (2005) A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest 128: 3810-3816.
-
McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, et al. (2007) Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 132: 1748-1755.
-
Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, et al. (2001) Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1414-1418.
-
Karadag F, Kirdar S, Karul AB, Ceylan E (2008) The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 19: 104-108.
-
Breen D, Churches T, Hawker F, Torzillo PJ (2002) Acute respiratory failure secondary to chronic obstructive pulmonary disease treated in the intensive care unit: a long term follow up study. Thorax 57: 29-33.
-
Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, et al. (2007) C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 250-255.
-
Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, et al. (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618-1623.
-
Xing Z, Braciak T, Jordana M, Croitoru K, Graham FL, et al. (1994) Adenovirus-mediated cytokine gene transfer at tissue sites. Overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol 153: 4059-4069.
-
Suwa T, Hogg JC, Klut ME, Hards J, van Eeden SF (2001) Interleukin-6 changes deformability of neutrophils and induces their sequestration in the lung. Am J Respir Crit Care Med 163: 970-976.
-
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, et al. (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645-2653.
-
Kuhn C 3rd, Homer RJ, Zhu Z, Ward N, Flavell RA, et al. (2000) Airway hyperresponsiveness and airway obstruction in transgenic mice. Morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J Respir Cell Mol Biol 22: 289-295.
-
Johnston RA, Schwartzman IN, Flynt L, Shore SA (2005) Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 288: 390-397.
-
Bathoorn E, Liesker JJ, Postma DS, Koëter GH, van der Toorn M, et al. (2009) Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis 4: 101-109.
-
Salvarani C, Casali B, Salvo D, Brunati C, Macchioni PL, et al. (1991) The role of interleukin 1, erythropoietin and red cell bound immunoglobulins in the anaemia of rheumatoid arthritis. Clin Exp Rheumatol 9: 241-246.
-
Markoulaki D, Kostikas K, Papatheodorou G, Koutsokera A, Alchanatis M, et al. (2011) Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Intern Med 22: 103-107.
-
Castell JV, Geiger T, Gross V, Andus T, Walter E, et al. (1988) Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem 177: 357-361.
-
McMahon G GF, Christopher K (2011) Chloride is associated with mortality in the critically ill. Crit Care Med 39: 96.
-
Gail Morrison (1990) Serum choloride. In: Walker HK, Hall WD, Hurst JW Clinical Methods: The history, physical, and laboratory examinations. 3rd edition Crit Care Med, Boston: Butterworths.
-
Pinto-Plata VM, Livnat G, Girish M, Cabral H, Masdin P, et al. (2007) Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest 131: 37-43.
-
Blasi F, Mantero M, Aliberti S (2012) Antibiotics as immunomodulant agents in COPD. Curr Opin Pharmacol 12: 293-299.
-
Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA (2000) Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 1608-1613.
-
Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Müllerova H, et al. (2007) Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 29: 527-534.
-
Malo O, Sauleda J, Busquets X, Miralles C, Agustí AG, et al. (2002) Systemic inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 38: 172-176.
-
Kersul AL, Iglesias A, Ríos Á, Noguera A, Forteza A, et al. (2011) Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 47: 176-183.
-
Ito K, Ito M, Elliott WM, Cosio B, Caramori G, et al. (2005) Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967-1976.
-
Ruiz-González A, Lacasta D, Ibarz M, Martínez-Alonso M, Falguera M, et al. (2008) C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology 13: 1028-1033.
-
Sin DD, Lacy P, York E, Man SF (2004) Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 170: 760-765.
-
Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, et al. (1997) Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol 17: 2167-2176.
-
Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, et al. (2012) Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 7: e37483.
-
Gross NJ (2012) Novel antiinflammatory therapies for COPD. Chest 142: 1300-1307.