International Journal of Respiratory and Pulmonary Medicine
Chronic Rhinosinusitis is Associated with Airflow Obstruction in Japanese Never-Smokers without Asthma
Keita Matsumoto1, Tomotaka Kawayama1*, Takashi Kinoshita1, Shuwa Minami2, Kazuko Matsunaga1,2, Masako Nagafuchi2, Tomoaki Hoshino1 and Toru Rikimaru2
1Division of Respirology, Neurology, and Rheumatology, Department of Medicine, Kurume University School of Medicine, Japan
2Respiratory Medicine, Kohokai Fukuoka Sanno Hospital, Japan
*Corresponding author: Tomotaka Kawayama, MD, PhD, Division of Respirology, Neurology, and Rheumatology, Department of Medicine, Kurume University School of Medicine, 67 Asahimachi, Kurume 830-0011, Japan, Tel: +81-942-31-7560, Fax: +81-942-31-7703, E-mail: kawayama_tomotaka@med.kurume-u.ac.jp
Int J Respir Pulm Med, IJRPM-2-023, (Volume 2, Issue 3), Original Article; ISSN: 2378-3516
Received: June 28, 2015 | Accepted: July 19, 2015 | Published: July 22, 2015
Citation: Matsumoto K, Kawayama T, Kinoshita T, Minami S, Matsunaga K, et al. (2015) Chronic Rhinosinusitis is Associated with Airflow Obstruction in Japanese Never-Smokers without Asthma. Int J Respir Pulm Med 2:023. 10.23937/2378-3516/1410023
Copyright: © 2015 Matsumoto K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The relationship between chronic rhinosinusitis (CRS) and airflow obstruction is still unclear. This cross-sectional study was conducted to investigate whether CRS is an independent risk factor for airflow obstruction in never-smokers without asthma.
Methods: One hundred fifty-eight subjects aged ≥ 40 yr without asthma were divided into 4 groups: 22 never-smokers with CRS, 27 smokers with CRS, 69 never-smokers without CRS, and 40 smokers without CRS. Subjects with airflow obstruction were compared among the groups, and the correlation between CRS severity and airflow obstruction was investigated. The presence of airflow obstruction was defined as a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of <0.7 and below the lower limit of normal (<LLN). Definitive diagnosis of CRS and its severity were based on clinical symptoms and findings of facial computed tomography.
Results: The proportion of patients with airway obstruction (FEV1/FVC ratio <0.7 and <LLN) was significantly higher among never-smokers (22.7% and 18.2%, p<0.05) and smokers (40.7% and 44.4%, p<0.05) with CRS than among those without CRS (4.3% and 4.3%, and 27.5% and 30.0%, respectively). The risk ratios (95% confidence interval) for CRS based on a FEV1/FVC ratio of <0.7 and <LLN were 3.29 (1.45-7.47, p<0.05) and 3.04 (1.35-6.82, p<0.05), respectively. The severity of CRS was significantly associated with the FEV1/FVC ratio (r=-0.23, p=0.0312) in never-smokers.
Conclusion: CRS is an independent risk factor for airflow obstruction in both smokers and never-smokers, and CRS severity is significantly associated with airflow obstruction in never-smokers. CRS may contribute to development of chronic obstructive pulmonary disease in never-smokers.
Keywords
Airflow limitation, Asthma, Chronic sinusitis, COPD, Lung function tests
Abbreviations
ATS: American Thoracic Society, CI: Confidence Interval, COPD: Chronic Obstructive Pulmonary Disease, CRS: Chronic Rhinosinusitis, CT: Computed Tomography, ERS: European Respiratory Society, FEV1: Forced Expiratory Volume in 1 second, FVC: Forced Vital Capacity, GOLD: Global Initiative for Chronic Obstructive Lung Disease, LLN: Lower Limit of Normal, SD: Standard Deviation, %FEV1: Percentage of Predicted Forced Expiratory Volume in 1 Second, %FVC: Percentage of Predicted Forced Vital Capacity
Introduction
Chronic rhinosinusitis (CRS) is characterized by mucosal inflammation affecting both the nasal cavity and paranasal sinuses [1,2]. CRS is one of the most common chronic upper respiratory tract conditions associated with chronic lower respiratory tract diseases such as prolonged and chronic cough, chronic bronchitis, cystic fibrosis, bronchiectasis, and asthma [1-7].
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction and pulmonary hyperinflation [8]. Cigarette smoke is the most important risk factor for development of COPD, but recently evidence of COPD in nonsmokers has been accumulating [9-12]. It is well known that a high proportion of patients with COPD have CRS as a comorbidity [13-16]. Asthma is an important factor related to the development of COPD in individuals who have never smoked [3-7]. However, the correlation between the presence of CRS and COPD in never-smokers without asthma is still unclear. Here we conducted a cross-sectional study to clarify whether CRS is an independent risk factor for airflow obstruction in never-smokers.
Methods
Ethical approval
The present study was conducted in accordance with good clinical practice guidelines and the declaration of Helsinki, and approved by the ethics committee of Fukuoka Sanno Hospital (no. FS-79, September 27th, 2012). Each investigator obtained written informed consent from all patients.
Subjects
Subjects aged ≥ 40 yr without asthma who presented at Fukuoka Sanno Hospital, Fukuoka, Japan, between October 2012 and June 2014 were recruited. Subjects who had a history of COPD and other chronic lower respiratory tract diseases, moderate to severe diseases of other organs such as heart failure, liver cirrhosis, renal failure, and cerebrovascular disease, or with active malignancies, were excluded. Subjects for whom flow-volume curves obtained by spirometry were inconclusive were also excluded. Patients with eosinophilic CRS. usually have bronchial asthma and peripheral eosinophilia. To remove any patients with asthma and/or eosinophilic CRS. those with blood eosinophil counts of >600/mm3 were excluded. Among our patients, 5, 6, 4, 1, and 17 received inhaled corticosteroids and long acting bronchodilators, anti-allergic agents, cystinyl leukotriene antagonists, macrolide antibiotics, and mucolytic agents, respectively, and none received intranasal or oral corticosteroids. However, all medications were washed out for at least 4 weeks after informed consent had been obtained.
Diagnosis of CRS and its severity
Diagnosis of CRS was based on symptoms such as nasal obstruction and discharge, discolored postnasal purulence and discharge, hyposmia or anosmia, and facial pain and pressure, which had persisted for >3 months. The features of sinuses revealed by facial computed tomography (CT) were also referred for diagnosis. Definitive diagnosis of CRS and nasal polyp was based on the opinions of two independent otolaryngologists.
The severity of CRS was based on the Lund-Mackay score, which ranges from 0 (complete lucency of all sinuses) to 24 (complete opacity of all sinuses) on facial CT [17]. Scores of 0 to 2 were rated as normal, and scores of 7 to 24 were rated as CRS. Patients with scores of 3 to 6 were excluded from the final analysis. The findings of facial and chest CT were interpreted by two independent radiologists.
Study design
This study had a cross-sectional design. After providing written informed consent, each subject was required to give medical information about symptoms, medication, smoking habits and smoking index (pack*yr) and comorbidities, and then blood tests, chest radiography, facial and chest CT, and spirometry were performed. Each subject was also required to meet the chest physicians and otolaryngologists after the examinations. None of the subjects were administered bronchodilators for spirometry.
Blood tests
Peripheral blood counts were measured using an automatic flow cytometry system (XE-5000, Sysmex co., Tokyo, Japan). Serum total IgE levels were measured using commercial fluorenzymeimmunoassay kits (SRL Inc., Tokyo, Japan). However, the serum specific IgE levels were not examined, as we were unable to obtain informed consent from all of the enrolled patients.
Spirometry
Spirometry was performed three times using an electronic spirometer (SP-770COPD, Fukuda Denshi, Tokyo, Japan) in accordance with the ATS/ERS task force document [18], and the best values of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were accepted. The diagnosis and severity of airflow obstruction were based on a FEV1/FVC ratio of <0.7 and the classification of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [7], respectively. We used the Japanese Respiratory Society (JRS) survey reference equations for Japanese men and women to calculate the predicted pulmonary function values [19]. The lower limit of the normal (LLN) threshold of the FEV1/FVC ratio was also evaluated as the lower fifth percentile of the predicted value (<LLN) based on the JRS survey for defining airflow obstruction [11,12,19].
Statistical analysis
The subjects were divided into 4 groups: never-smokers with CRS, smokers with CRS, never-smokers without CRS, and smokers without CRS. All the data were expressed as mean ± standard deviation (SD). The characteristics of the subjects were compared using Student's t test and Χ2 test. The risk ratios [95% confidence interval (CI)] for CRS based on airflow obstruction were assessed using univariate and multivariate analyses. The correlations (r) between predicted %FEV1 and the severity of chronic sinusitis, age, and smoking index were analyzed by nonparametric Spearman's test. Differences at p <0.05 were considered statistically significant. All analyses were performed using the statistical software package JMP version 9.0® (SAS Institute Japan Inc., Tokyo, Japan).
Results
Characteristics of the subjects
As shown in figure 1, 228 subjects aged ≥ 40 yr without asthma were enrolled. However, six subjects with bronchiectasis defined by chest CT were excluded, as were 15 subjects who did not provide details of their smoking history. Fourteen subjects with blood eosinophil counts of >600/mm3 were also excluded. An additional 35 subjects were excluded because a definitive diagnosis of CRS and normal sinus could not be obtained. Thus, 158 subjects were finally analyzed. The numbers (%) of subjects with and without definitive CRS were 49 (31.0) and 109 (69.0), respectively. There were 22 never-smokers with CRS, 27 smokers with CRS, 69 never-smokers without CRS, and 40 smokers without CRS.
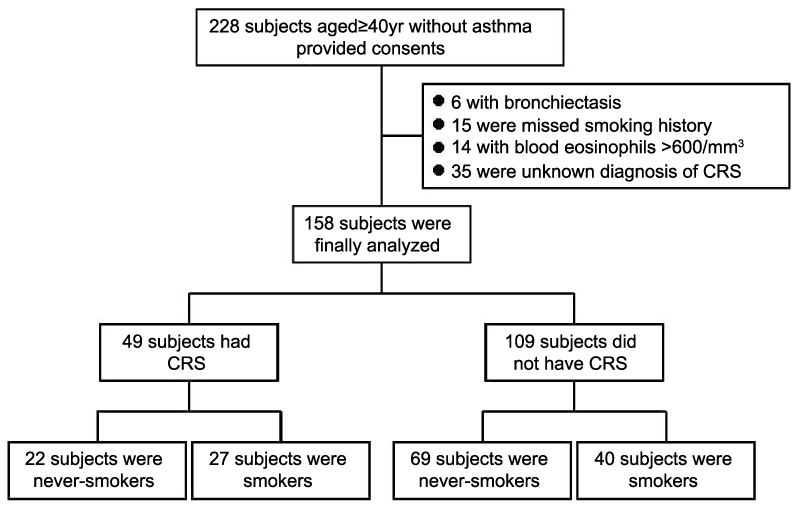
.
Figure 1: Study design
Written informed consent was obtained from 228 subjects aged ≥ 40 yr without asthma. Six subjects with bronchiectasis were excluded, as were 15 for whom data on smoking history were unavailable. Fourteen patients with blood eosinophil counts of >600/mm3 were also excluded. Thirty-five subjects who were not definitively diagnosed as having CRS or normal sinus were also excluded.
CRS: Chronic Rhinosinusitis, LLN: Lower Limit of Normal, FEV1: Forced Expiratory Volume in 1 second, FVC: Forced Vital Capacity
View Figure 1
Table 1 shows the characteristics of the subjects. Males accounted for a significantly higher proportion of smokers with and without CRS than among never-smokers. Five (22.7%) never-smokers and 3 (11.1%) smokers with CRS had nasal polyps. Smokers with CRS had a significantly lower FEV1/FVC ratio than never-smokers with CRS (p=0.0273) or never-smokers without CRS (p<0.001). Smokers without CRS had a significantly lower FEV1/FVC ratio than never-smokers without CRS (p=0.0153).
![]()
Table 1: Characteristics of the subjects
View Table 1
The proportion (%, p value) of never-smokers with CRS [5 (22.7%, p=0.0265)] who had a FEV1/FVC ratio of <0.7 was significantly higher than that of never-smokers without CRS [3 (4.3%)], whereas there was no difference in the proportion of <LLN patients between these two groups [4 (18.2%) and 3 (4.3%), respectively] (p=0.0967). Among smokers there was no difference in the proportion of patients with a FEV1/FVC ratio of <0.7 and <LLN patients between the CRS [11 (40.7%, p=0.3860) and 12 (44.4%, p=0.3422), respectively] and non-CRS [11 (27.5%) and 12 (30.0%), respectively] groups. The numbers of subjects who had a stage II or more severe GOLD classification with a FEV1/FVC ratio of <0.7 and <LLN were 5 and 4 for never-smokers with CRS, 9 and 10 for smokers with CRS, 3 and 3 for never-smokers without CRS, and 9 and 10 for smokers without CRS, respectively (Table 1).
The subjects with CRS had significantly higher blood eosinophil counts than those without CRS (p<0.0001). In never-smokers and smokers, there was no significant correlation between blood eosinophil counts and %FEV1 (r=-0.1, p>0.05 and r=-0.1, p>0.05, respectively) and the FEV1/FVC ratio (r=-0.0, p>0.05 and r=-0.1, p>0.05, respectively), respectively. There were no significant differences in the serum total IgE levels among the 4 groups (Table 1).
Univariate analysis of risk factors for airflow obstruction
Univariate analysis showed that the risk ratios (95%CI, p value) for the presence of CRS were 3.29 (1.45-7.47, p<0.05) and 3.04 (1.35-6.82, p<0.05) for patients aged ≥ 70 yr, 3.77 (1.49-9.55, p<0.05) and 2.84 (1.11-7.24, p>0.05) for male patients, 4.36 (1.75-10.9, p<0.05) and 5.81 (2.23-15.2, p<0.01) for cigarette smoking, and 5.07 (2.09-12.3, p<0.05) and 6.70 (2.670-16.8, p<0.0001) for a FEV1/FVC ratio of <0.7 and <LLN (Table 2). The presence of nasal polyps was not a risk factor (p>0.05 for both a FEV1/FVC ratio of <0.7 and <LLN).
![]()
Table 2: The risk ratios of parameters associated with airflow obstruction by univariate analysis
View Table 2
Multivariate analysis of risk factors for airflow obstruction
Multivariate analysis showed that the adjusted risk ratios (95%CI, p value) for the presence of CRS were 2.65 (1.09-6.53, p<0.05) for patients aged ≥ 70 yr, 3.45 (1.22-9.74, p<0.05) for male patients, 1.86 (0.61-5.90, p>0.05) for cigarette smoking, and 3.20 (1.13-9.82, p<0.05) for a FEV1/FVC ratio of <0.7, whereas the corresponding ratios for <LLN were 2.46 (1.02-5.96, p<0.05), 2.64 (0.91-7.47, p>0.05), and 5.90 (2.40-16.2, p<0.0001), respectively (Table 3).
![]()
Table 3: The adjusted risk ratios of parameters associated with airflow obstruction by multivariate analysis
View Table 3
Correlation between Lund-Mackay scores for CRS and airflow obstruction
The Lund-Mackay scores for CRS were significantly associated with the FEV1/FVC ratio (r=-0.23, p=0.0312), but not with the predicted %FEV1 (r=-0.20, p=0.0615) in never-smokers, whereas in smokers the scores were significantly associated with the predicted %FEV1 (r=-0.25, p=0.0417), but not with the FEV1/FVC ratio (r=-0.22, p=0.0785).
Discussion
In this Japanese cohort, we have demonstrated that the presence of CRS is an independent risk factor for development of airflow obstruction in never-smokers aged ≥ 40 yr without asthma. Another recent Japanese study also showed that CRS patients had significant airflow obstruction regardless of the presence of asthma [7]. In our present study, about 30% of the 158 subjects with prolonged cough had a definitive diagnosis of CRS. We considered airflow obstruction to be present in patients who had a FEV1/FVC ratio of <0.7 and <LLN. Seventeen (34.7%) and 16 (32.7%) of the 49 subjects with CRS had a FEV1/FVC ratio of <0.7 and <LLN, respectively. Thirty (19.0%) and 31 (19.6%) of the 158 subjects had a FEV1/FVC ratio of <0.7 and <LLN, respectively, without administration of bronchodilators. Among never-smokers and smokers with CRS, 5 (22.7%) and 4 (18.2%), respectively, had airflow obstruction based on a FEV1/FVC ratio of <0.7 and <LLN. All of the never-smokers evaluated as having airflow obstruction based on both of the latter criteria were GOLD stage II. The different results obtained using these two criteria were statistically close. Previous studies had demonstrated that 2-8% of never-smokers in the general Japanese population had airflow obstruction [20-22]. Although the data were not directly comparable, the proportion of never-smokers with CRS who had airflow obstruction in our study was higher than that in the general population. Although the presence of nasal polyps might be one of the risk factors for airflow obstruction [19], this was not the case in the present study because the proportion of subjects with nasal polyps was small. It is well known that CRS with nasal polyps is associated with asthma and adverse reactions to aspirin and other non-steroidal anti-inflammatory drugs that inhibit cyclooxygenase-1. Therefore, CRS with nasal polyps is thought to be mainly characterized by eosinophilic inflammation and prevalent Th2 responses including release of IL-5, but not neutrophilic types of CRS [23]. Thus there may be differences between eosinophilic or neutrophilic CRS and with airflow obstructions, and further analysis will be needed to clarify this issue.
We also found that the severity of CRS was significantly associated with the FEV1/FVC ratio in never-smokers, whereas in smokers the severity was significantly associated with predicted %FEV1. In addition, multivariate analysis showed that the presence of CRS, smoking history, and higher age were independent risk factors for airflow obstruction.
Interaction between asthma and CRS has been well documented in previous studies [24,25]. In the present study, we carefully excluded subjects with chronic lower respiratory diseases associated with CRS, such as asthma, bronchiectasis and interstitial pneumonia. Using chest CT, our two independent radiologists found signs of bronchiectasis in 6 patients. None of the subjects had interstitial pneumonia, but emphysematous changes were evident in some smokers. Our results demonstrated that never-smokers with CRS included a significantly higher proportion (2.46- to 2.65-fold) of individuals with airflow obstruction than never-smokers without CRS.
The study had some limitations. First, although all of the subjects had normal chest CT findings, pathohistological features in the bronchi and bronchioles were not assessed. In order to investigate the interactions between airflow obstruction and CRS, it will be necessary to study airway inflammation and hyperresponsiveness, and the reversibility of airway obstruction after administration of bronchodilators. In this study we demonstrated that subjects with CRS, both never-smokers and smokers, had significantly higher peripheral eosinophil counts than subjects without CRS, even though we excluded patients with asthma via interview and determination of blood eosinophil counts (>600/mm3). Second, interactions between CRS symptoms, rhinorrhea inflammation, and airway obstruction remained unclear [26]. Third, we did not investigate whether control of CRS would improve airflow obstruction. Further intervention trials will therefore be necessary to clarify these issues.
In summary, the present study has shown that CRS is an independent risk factor for development of airflow obstruction, and that the severity of CRS is significantly associated with airflow obstruction in never-smokers. We believe that, at least in Japanese patients, the presence of CRS contributes to chronic obstructive pulmonary disease in never-smokers.
Acknowledgements
The authors extend special thanks to Dr. Toshiro Umezaki, MD, PhD, and Dr. Hideyuki Kiyohara, MD, PhD, Fukuoka Sanno Hospital, for diagnosis of CRS. The authors are also grateful to Dr. Hidetsuna Utsunomiya, MD, PhD, and Dr. Shuji Kaneko, MD, PhD, for reviewing the facial and chest CT films. The authors also thank Mrs. Kyoko Yamaguchi, BSc, and Mrs. Toshie Ikari, BSc, Kurume University, for management of the study data. The authors are also grateful to Professor Howard A. Young, PhD, National Cancer Institute-Frederick, for English support during preparation of the manuscript.
References
-
Marple BF, Stankiewicz JA, Baroody FM, Chow JM, Conley DB, et al. (2009) Diagnosis and management of chronic rhinosinusitis in adults. Postgrad Med 121: 121-139.
-
Rosenfeld RM (2007) Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg 137: 365-377.
-
Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, et al. (2004) The diagnosis and management of chronic cough. Eur Respir J 24: 481-492.
-
Guilemany JM, Angrill J, Alobid I, Centellas S, Pujols L, et al. (2009) United airways again: high prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy 64: 790-797.
-
Hayden FG (2004) Rhinovirus and the lower respiratory tract. Rev Med Virol 14: 17-31.
-
Vinuya RZ (2002) Upper airway disorders and asthma: a syndrome of airway inflammation. Ann Allergy Asthma Immunol 88: 8-15.
-
Kariya S, Okano M, Higaki T, Noyama Y, Haruna T, et al. (2014) Chronic rhinosinusitis patients have decreased lung function. Int Forum Allergy Rhinol 4: 828-833.
-
(2014) Global initiative for chronic obstructive lung disease. Global strategy for diagnosis, management, and prevention of COPD.
-
Thomsen M, Nordestgaard BG, Vestbo J, Lange P (2013) Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 1: 543-550.
-
Waked M, Salame J, Khayat G, Salameh P (2012) Correlates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional study. Int J Chron Obstruct Pulmon Dis 7: 577-585.
-
Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, et al. (2011) COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 139: 752-763.
-
Bridevaux PO, Probst-Hensch NM, Schindler C, Curjuric I, Felber Dietrich D, et al. (2010) Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J 36: 1259-1269.
-
Halawi AM, Smith SS, Chandra RK (2013) Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc 34: 328-334.
-
Gonem S, Raj V, Wardlaw AJ, Pavord ID, Green R, et al. (2012) Phenotyping airways disease: an A to E approach. Clin Exp Allergy 42: 1664-1683.
-
Piotrowska VM, Piotrowski WJ, Kurmanowska Z, Marczak J, Gorski P, et al. (2010) Rhinosinusitis in COPD: symptoms, mucosal changes, nasal lavage cells and eicosanoids. Int J Chron Obstruct Pulmon Dis 5: 107-117.
-
Gliklich RE, Metson R (1995) The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 113: 104-109.
-
Oluwole M, Russell N, Tan L, Gardiner Q, White P (1996) A comparison of computerized tomographic staging systems in chronic sinusitis. Clin Otolaryngol Allied Sci 21: 91-95.
-
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319-338.
-
Toda R, Hoshino T, Kawayama T, Imaoka H, Sakazaki Y, et al. (2009) Validation of "lung age" measured by spirometry and handy electronic FEV1/FEV6 meter in pulmonary diseases. Intern Med 48: 513-521.
-
Omori H, Nagano M, Funakoshi Y, Onoue A, Mihara S, et al. (2011) Twelve-year cumulative incidence of airflow obstruction among Japanese males. Intern Med 50: 1537-1544.
-
Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, et al. (2004) Clinical phenotypes of COPD: results of a Japanese epidemiological survey. Respirology 9: 331-336.
-
Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, et al. (2004) COPD in Japan: the Nippon COPD Epidemiology study. Respirology 9: 458-465.
-
Ishitoya J, Sakuma Y, Tsukuda M (2010) Eosinophilic chronic rhinosinusitis in Japan. Allergol Int 59: 239-245.
-
Feng CH, Miller MD, Simon RA (2012) The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 26: 187-190.
-
Pakdaman MN, Luong A (2011) The links between chronic rhinosinusitis and asthma. Curr Opin Otolaryngol Head Neck Surg 19: 218-223.
-
Piccirillo JF, Merritt MG Jr, Richards ML (2002) Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126: 41-47.





