International Journal of Ophthalmology and Clinical Research
Aflibercept for the Treatment of Recalcitrant Macular Degeneration: Results from a One Year Prospective Cohort Study. The Auckland Experience
David Squirrell1,2, Priya Samalia1,2*, Leo Sheck1, Rachel Barnes1,3 and Dianne Sharp1,3
1Greenlane Clinical Centre, Epsom, Auckland, New Zealand
2Department of Ophthalmology, University of Auckland, Grafton, Auckland, New Zealand
3Retina Specialists, Parnell, Auckland, New Zealand
*Corresponding author: Priya Samalia, Department of Ophthalmology, University of Auckland, Grafton, Auckland, New Zealand, 201 Great King Street, Dunedin, New Zealand, Tel: +64 3 474 7970, E-mail: prapri14@gmail.com
Int J Ophthalmol Clin Res, IJOCR-3-053, (Volume 3, Issue 2), Research Article; ISSN: 2378-346X
Received: March 13, 2016 | Accepted: May 20, 2016 | Published: May 23, 2016
Citation: Squirrell D, Samalia P, Sheck L, Barnes R, Sharp D (2016) Aflibercept for the Treatment of Recalcitrant Macular Degeneration: Results from a One Year Prospective Cohort Study. The Auckland Experience. Int J Ophthalmol Clin Res 3:053. 10.23937/2378-346X/1410053
Copyright: © 2016 Squirrell D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To investigate the effect of switching patients with neovascular age related macular degeneration (nAMD) non-responsive to bevacizumab or ranibizumab to intravitreal aflibercept 2 mg on best corrected visual acuity, macula volume, and central macula thickness.
Methods: 50 patients with nAMD non-responsive to bevacizumab or ranibizumab received 2 mg intravitreal aflibercept, at three 4-weekly doses, then every 8 weeks for 48 weeks. Primary outcome was BCVA at week 48 as measured on ETDRS charts. Secondary outcomes were proportion of patients with no fluid on OCT at week 12, 24 and 48; BCVA at week 12 and 24; mean changes in central macula thickness and macular volumes at week 12, 24 and 48; and tolerability and safety of aflibercept.
Results: There was a trend towards an improvement of BCVA at week 48 compared to baseline (Student's t-test p = 0.056, Wilcoxon signed-ranked test p = 0.09901). There was a statistically significant improvement in central macula thickness (-99 μm, p < 0.001), macular volume (-0.75 mm3 p < 0.001) and PED height (-52.5 μm p < 0.001). 62% of these patients with previous recalcitrant disease had complete resolution subretinal fluid at the close of the study.
Conclusion: Intravitreal aflibercept is a potentially viable treatment strategy for patients with recalcitrant neovascular AMD non-responsive to anti-VEGF monoclonal antibodies.
Keywords
Recalcitrant age-related macular degeneration, Aflibercept, Polypoidal choroidal vasculopathy, VEGF (vascular endothelial growth factor)
Abbreviations
AFL: Aflibercept, AMD: Age related Macular Degeneration, nAMD: Neovascular Age related Macular Degeneration, BCVA: Best Corrected Visual Acuity, CI: Confidence Interval, CMT: Central Macula Thickness, CNV: Choroidal Neovascular Membrane, ETDRS: Early Treatment Diabetic Retinopathy Study, IOP: Intraocular Pressure, IQR: Interquartile Range, IVT: Intravitreal, OCT: Ocular Coherent Tomography, PED: Pigment Epithelial Detachment, PCV: Polypoidal Choroidal Vasculopathy, PIGF: Placental growth Factor, prn: Pro Re Nata, RAP: Retinal Angiomatous Proliferation, RPE: Retinal Pigment Epithelium, VEGF: Vascular Endothelial Growth Factors.
Introduction
The introduction of the anti-VEGF agents into clincial practice has radically changed the outlook for patients diagnosed with neovascular age related macular degeneration (nAMD). Currently three agents are available in clinical practice; bevacizumab, ranibizumab, and aflibercept. At the present time the debate continues as to which of these agents represents a cost effective first line agent but it would appear from the key note non-inferiority comparative trials that if there is a difference between these agents it may be, for most patients, a modest one [1-3]. At the time this study was conducted bevacizumab was funded in New Zealand as first line treatment of nAMD with ranibizumab being restricted for use as a second line agent in those patients who were deemed to have an inadequate response to this agent in their "only seeing eye". Aflibercept remains unfunded.
Regardless of the agent used it is increasingly clear that a significant minority of patients treated with a given anti-VEGF agent have a poor or inadequate response to treatment and a number of definitions have now emerged in the literature for describing these recalcitrant lesions. In broad terms recalcitrant nAMD can be divided into those patients who are "non responders"; those whose disease fails to respond to treatment from the outset; or tachyphylaxis, where there was initially a favourable respose to anti-VEGF therapy, which waned with ongoing therapy. To complicate matters "nonresponders" have also been defined on functional criteria; patients vision fails to improve with treatment [4], and anatomical criteria; failure of the subretinal or intraretinal fluid to resolve [5-12]. This current study was designed to assess the efficacy of aflibercept in treating patients whose nAMD had failed to respond to, or who had become non-responsive to the currently funded agents namely bevacizumab and ranibizumab. In this study, we used anatomical criteria to define the lack of response - persistent intraretinal or subretinal fluid 28 days after a minimum of four consecutive ranibizumab and/or bevacizumab injections [6]. This study reports the 1 year outcome data after switching patients with recalitrant nAMD to intravitreal aflibercept (IVT-AFL) 2 mg.
Methods
Participants
Patients were recruited from two practices in Auckland. One a private clinic: Retina Specialists (Parnell, Auckland, New Zealand) and the public sector macular service at Auckland District Health Board (Auckland, New Zealand). The inclusion and exclusion criteria are summarized in table 1. The mean age of patients was 78 years with a slight female preponderance (58%). The target patients were those with nAMD who were "non-responsive or partially responsive" to either bevacizumab or ranibizumab as defined as: having received at least 4 injections of either or both anti-VEGF agents in the past 6 months and despite this had persistent intraretinal or subretinal fluid, or both, on spectral-domain optical coherence tomography (OCT) on all occasions throughout this treatment period. The cohort of patient recruited into this study includes patients who had been treated with bevacizumab monotherapy as well as patients whose "only seeing eye" was affected and who had therefore been treated with both bevacizumab and ranibizumab.
![]()
Table 1: Inclusion and exclusion criteria.
View Table 1
Informed consent process
All eligible patients were approached and invited to participate in the study. Patients were provided with both verbal and written information on the study. Verbal and written consent was obtained from each patient. Patients were able to exit the study at any time point. The tenants of the declaration of Helsinki were followed.
Interventions
All patients received three IVT-AFL injections 4 week apart commencing 6 weeks after their last intravitreal anti-VEGF injections in the study eye. Further injections were given every 8 weeks (at week 16, 24, 32, 40). The study terminated at week 48 when an exit review was completed. The IVT-AFL injections were given using sterile technique in a dedicated operating room. Following topical anesthesia with amethacaine drops, 5% iodine was used for surface sterilization. After placement of a lid speculum 2 mg of IVT-AFL in 0.05 ml (Bayer, Germany) was injected at the pars plana, 3.5 to 4 mm posterior to the limbus, into the vitreous cavity. The patency of the central retinal artery was verified in all patients following injection, and ocular lubricant was prescribed following the procedure.
Outcome measures
In all patients, best corrected refracted visual acuity (BCVA) as measured on 6 meter ETDRS chart, OCT (Spectralis, Heidelberg Engineering, Germany), and IOP (Goldmann tonometry) were recorded every 4 weeks. Contrast sensitivity was assessed at baseline, 6 months and 12 months. All assessments were standardized across the two study sites. In a subgroup of 36 patients; those who were recruited from the public sector macular service at Auckland District Health Board (Auckland, New Zealand); total macular volume was also assessed using the Heidelberg Eye Explorer volume analysis software inherent in the thickness map report (Heidelberg, Germany) after manually adjusting the segmental lines to include the basement membrane to the internal limiting membrane in all 19 scanned OCT images of the central macular field. Pigment epithelial detachment (PED) volume and height was similarly measured by adjusting the segmental lines appropriately.
The primary outcome measure was BCVA at week 48. Secondary outcomes were proportion of patients with no fluid on OCT at week 12, 24 and 48; BCVA at week 12 and 24; mean changes in central macula thickness (CMT), mean change in macular volumes at week 12, 24 and 48; mean change in PED height and volume compare to baseline, change in contrast sensitivity relative to baseline at weeks 24 and 48, and tolerability and safety of IVT-AFL.
Statistical analysis
The proportion of those patients whose visual acuity improved after 12 months was compared to baseline. A similar analysis was conducted to assess the proportion of patients whose disease activity (as measured by macular volume, CMT, PED volume and height and resolution of sub retinal fluid) improved compared to baseline.
Baseline data was assessed for normality using the Shapiro-Wilk statistical analysis. BCVA and macula volume were found to be normally distributed. CMT, PED volume and PED height were not normally distributed.
For normally distributed data (BCVA, macula volume) at each time period, including the baseline, the mean of the outcome measure along with 95% confidence interval is presented. For data that did not follow a normal distribution (CMT, PED volume, PED height), at each time period, including the baseline, the median of the outcome measure along with interquartile range (IQR) is presented.
The Wilcoxon signed-rank test and the Student's t-test for paired samples were used to test for differences in outcome measures between each time period and the baseline as appropriate. P-values for each test are presented and value < 0.05 was deemed statistically significant.
Sample size
From our local audit data, it was found that approximately 8-10% of patients with nAMD did not respond adequately to the currently funded anti-VEGF agents. With a total cohort of 700 patients in the two services, it was estimated that approximately 70 patients were therefore being inadequately treated with the existing treatments. As this study was an investigator led trial, a proposal was made to Bayer New Zealand to conduct a trial comprising 50 patients. Such an approach not only secured access to aflibercept for most of our eligible patients but it also ensured that we had as large a sample size as practically possible.
Results
Demographic data
50 patients were recruited for this study (Table 2). There was a slight female predominance. The mean duration of disease prior to the patient being recruited into the study and receiving Aflibercept treatment was 35 months. The majority of lesions were occult choroidal neovascular membrane (CNV), with a smaller proportion of classic CNV, polypoidal choroidal vasculopathy (PCV), and retinal angiomatous proliferation (RAP). Mean baseline BCVA was 71 letters. 48 patients completed the study, with one death from an unrelated cause (pancreatic cancer) and one withdrawal at week 24.
![]()
Table 2: Demographic Data.
View Table 2
Prior to their recruitment into this study patients had received a median of 22 anti-VEGF intraviteal injections in the study eye (bevacizumab and/or ranibizumab) (Table 2). The initial treatment of the nAMD in all but two patients was bevacizumab with 27 patients having recieving bevacizumab monotherapy alone before entry into the study. These patients had been managed with avastin monotherapy, despite having repeatedly demonstrated a lack of efficacy, as they could not secure fundng to support a switch to ranibizumab treatment. In this subgroup of patients the mean time that had elapsed between the disease presenting and the patient being treated with aflibercept was 32 months. In 21 patients the treatment had been switched from bevacizumab to ranibizumab prior to entry into the study. The time of the switch from bevacizumab to ranibizumab in this cohort of patients was determined by how long it took for the individual to secure public funding to pay for this treatment once it had been estabished that avastin monotherapy was not effectively treating their disease. In this subgroup of patients the mean time that had elapsed between their switch to ranibizumab and their first treatment with aflibercept was 7 months (Table 2). Two patients had been managed solely with ranibizumab monotherapy prior to entry into the study.
For the total cohort of 50 patients the median number of bevacizumab injections administered prior to entry into the study total was 17 and the median number of ranibiumab injections administered was zero (Table 2). The latter figure is a reflection on that the fact that a majority of patients (27) received avastin monotherapy and thus received 0 ranibizumab injections. The median number of ranibizumab injections administered to those patients (23) who were treated with ranibizumab at some point prior to recruitment into the study was 7.
Primary outcome
Mean BCVA was measured as 71 (95% CI: 67, 74) letters at week 12, 72 (95% CI: 70, 74) letters at week 24, and 72 (95% CI: 70, 75) letters a week 48. As compared to baseline, there was a trend to an improvement of BCVA at week 48 but this did not achieve statistical significance at the 5% level (Student's t-test p-value = 0.057, Wilcoxon signed-rank test p-value = 0.09901; Figure 1).
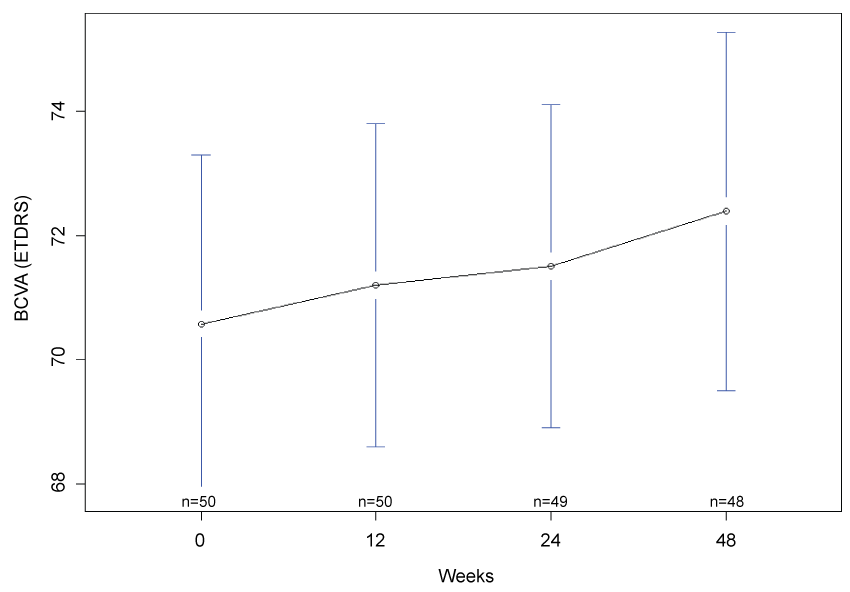
.
Figure 1: Mean best corrected visual acuity at baseline, 12 weeks, 24 weeks and 48 weeks. There was a trend towards improvement in BCVA (Student's t-test p = 0.056, Wilcoxon signed-rank test p = 0.09901) at the closure of the study. n denotes the number of paticipants completing follow-up at each time point and error bars indicate 95% confidence interval.
View Figure 1
Secondary outcomes
Central macula thickness: There was a statistically significant decrease in median CMT at week 12 (306.5 μm, IQR: 260, 382.5, p < 0.001), week 24 (348 μm, IQR: 310.5 μm, 438 μm, p < 0.001) and week 48 (325 μm, IQR: 284.5 μm, 423.8 μm, p < 0.001), as compared to baseline (424 μm, IQR: 336.8 μm, 495.5 μm) (Table 3).
![]()
Table 3: Median central macula thickness at baseline, 12 weeks, 24 weeks and 48 weeks. There was a statistically significant improvement in central macula thickness (P < 0.001) at the closure of the study. n denotes the number of paticipants completing follow-up at each time point.
View Table 3
Macula volume: There was a statistically significant decrease in mean macula volume at week 12 (7.9 mm3 , 95% CI: 7.5 mm3, 8.3 mm3, p < 0.001), week 24 (8.17 mm3, 95% CI: 7.76 mm3, 8.58 mm3, p < 0.001) and week 48 (8.0 mm3, 95% CI: 7.6 mm3, 8.3 mm3, p < 0.001), as compare to baseline (8.8 mm3, 95% CI: 8.3 mm3, 9.2 mm3) (Figure 2).
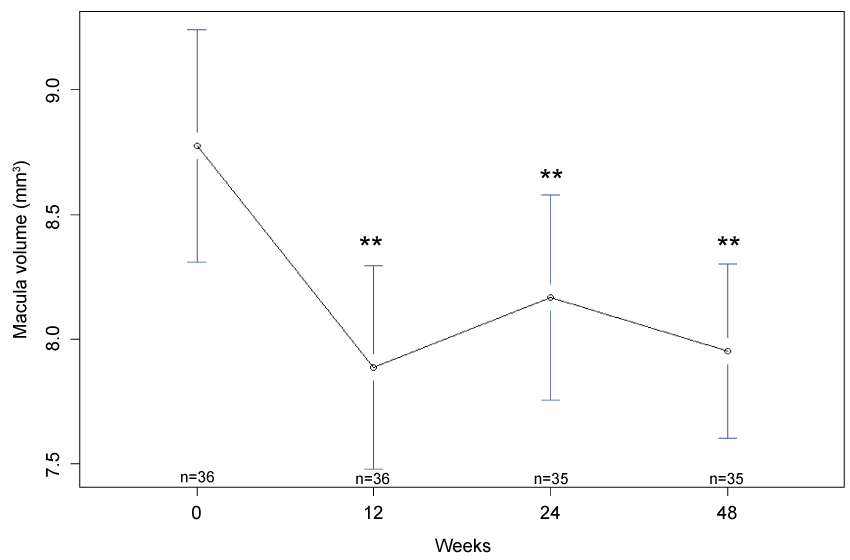
.
Figure 2: Mean macular volume at baseline, 12 weeks, 24 weeks and 48 weeks. There was a statistically significant improvement in macular volume (**p < 0.001) at the closure of the study. n denotes the number of paticipants completing follow-up at each time point and error bars indicate 95% confidence interval.
View Figure 2
PED volume and height: Of the 36 patients who underwent additional macular volume analysis 32 had a PED associated with their nAMD. There was a statistically significant decrease in the median height and volume of these PED at weeks 12 (height 156 μm, IQR: 103, 287, P < 0.001; volume 0.79 mm3, IQR: 0.46, 1.10, Student's t-test 0.003, Wilcoxon signed-rank test 0.002) and weeks 48 (height 157 μm, IQR: 85, 229.5, Student's t-test 0.0002, Wilcoxon signed-rank test p 0.0002; and volume 0.67 mm3, IQR: 0.48, 1.08, Student's t-test p = 0.005, Wilcoxon signed-rank test p = 0.001) as compared to baseline (height 209.5 μm, IQR: 111.5, 325.8; volume 0.905 mm3, IQR: 0.57, 2.13) (Table 4). Of these 32 patients; 18 experienced an improvement in the size of the PED at the close of the study and in 2 patients the PED resolved completely. In all cases where the PED did not respond to treatment the PED was classified as solid with hyperreflective or mixed elements [13].
![]()
Table 4: Spectral domain optical coherence tomography analysis of pigment epithelial detachment after switching to aflibercept in eyes with refractory neovascular age-related macular degeneration (n = 36).
View Table 4
Contrast sensitivity: There was no significant change in contrast sensitivity compared to baseline at any of the time points tested (at 6 months compared to baseline Student's t-test p = 0.6219, Wilcoxon signed-rank test p = 0.5116; at 12 months compared to baseline Student's t-test p = 0.6972, Wilcoxon signed-rank test p = 0.4626).
Adverse events: No significant adverse events were observed and no patient required cataract surgery during the study period. Two cases of subconjunctival haemorrhage were observed at the injection site and 5 patients reported pain after the injection lasting less than 12 hours at some time point in the study. One patient passed away from a late presenting pancreatic adenocarcinoma.
Discussion
Currently, there is limited evidence to guide clinicians in the management of patients who show an inadequate response to first line anti-VEGF treatment. In broad terms clinicians have explored two approaches to deal with this increasingly recognised issue; super dose regimens or switching regimens. It has previously been hypothesized that patients whose nAMD proved recalcitrant to treatment with conventional dosing regimens of bevacizumab and ranibizumab may not have been at the peak of their dose-response curve. Whilst the HARBOR trial concluded that in treatment naive patients "super dosing" with 2.0 mg ranibizumab conferred no additional benefit compared to conventional dosing regimens it did not address the question of whether a super dose regimen would improve efficacy in that subset of patients who were "non-responders" to conventional therapy [14]. Consequently, the SAVE study was designed with the intention of assessing whether a "super-dose" 2.0 mg ranibizumab regimen would be an effective strategy for treating those patients who failed to respond to conventional dose regimens. The 3 month data from the SAVE trial initially suggested that greater VEGF blockade may indeed have a beneficial role in the management patients with recalcitrant nAMD. When treated with fixed dose monthly 2.0 mg ranibizumab patients gained a modest improvement in visual acuity and macular thickness [15]. Although the modest gain in visual acuity was maintained after switching to a "prn" protocol at the end of year 2 the majority of patients continued to require intensive treatment with 70% of patients receiving all possible treatments because of persistent fluid and the 2.0 mg dosage was subsequently withdrawn [16]. Similarly disappointing results were reported in the LAST study [17]. At the close of this small pilot study comprising 7 patients who received 2.0 mg ranibizumab 71% had persistent subretinal fluid and 57% had persistent intraretinal fluid. Moreover, the percentage of patients whose PED volume and sub/ intra retinal fluid worsened over the 12 months exceeded the percentage of patients in whom these parameters improved [17]. Overall, these data would suggest that the "super-dose" strategies have little role in the management of patients with nAMD which is recalcitrant to conventional dosing regimens.
Switching anti-VEGF agents is increasingly being advocated for the management of patients with recalcitrant nAMD. Interpreting the "switching" literature can however be confusing as authors use different definitions for what represents a treatment "non responder." Whereas some authors use functional criteria [4]; failure of the visual acuity to improve, others use an anatomical definition [5-12]; persistence of sub/intra retinal fluid and yet others use both [18]. The differing inclusion criteria will naturally influence the study population which means one has to exercise caution when interpreting the data from such studies. With this caveat the results of studies which have switched patients from ranibizumab to bevacizumab or vice versa have largely been disappointing.
In a large retrospective review of functional non-responders, those patients who were switched from bevacizumab to ranibizumab had a small but significant improvement in BCVA and CMT (-66 μm) but patients that switched from ranibizumab to bevacizumab had no significant improvement in either of these parameters. Linear regression analysis revealed that visual acuity at the time of the switch was the factor most strongly correlated with a subsequent improvement in visual acuity. Interestingly, the group that switched from bevacizumab to ranibizumab had a significantly better visual acuity at baseline prior to the switch compared to the group that switched from ranibizumab to bevacizumab [4]. Although, this finding has not been replicated in other studies this finding suggests that when dealing with patients with recalitrant disease one should be prepared to switch agents early if the best outcome is to be achieved for the patient. A more recent retrospective review of 110 patients who were switched from bezacizumab to ranibizumab reported that, at month 3, switching anti-VEGF agents failed to lead to an improvement in visual acuity but did lead to a statistically significant but a modest; 31.0 μm, improvement in central retinal thickness [18]. However, this improvement was not maintained to the end of follow-up (mean 14 months). Unfortunately no data was reported as to the proportion of patients whose subretinal/intraretinal fluid resolved after switching agents in either of these two studies [4,18]. These data, as well as the data from the large keynote papers comparing ranibizumab and bevacizumab [1,2] suggest that, perhaps at best, only a modest benefit may be expected after switching between these two agents.
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to and non-specifically inhibits all VEGF-A isoforms. Similarly, ranibizumab is a recombinant humanized monoclonal antibody fragment that inhibits VEGF-A. Unlike ranibizumab and bevacizumab, aflibercept binds all isoforms of VEGF-A as well as VEGF-B and placental growth factor (PIGF), potentially making this agent more efficacious [19]. Aflibercept is a soluble decoy receptor based on "Trap" technology that binds to both sides of the VEGF dimer forming a complex [20,21]. Moreover it also has a significantly higher binding affinity for VEGF compared to bevacizumab and ranibizumab [22,23]. It has therefore been hypothesised that aflibercept may more effectively treat patients whose nAMD was previously recalcitrant to treatment with ranibizumab or bevacizumab. A number of retrospective case series have emerged which lend weight to this hypothesis [5-7,9-12,24-26], with improvements in visual acuity and central macular thickness being reported after patients with recalitrant nAMD were switched to aflibercept.
The results from these retrospective case series have been corroborated by the 4 prospective studies that have examined the efficacy of switching to aflibercept from bevacizumab or ranibizumab in patients with recalcitrant nAMD [8,16,27-29]. The results from our current study broadly support the published data. Of the currently published prospective "switch" trials the TURF trial [30] is the least representative of current practice as the participants in this study were recruited from the "super dose" SAVE trial. After 6 months of treatment with aflibercept there was no improvement in visual acuity and only a modestly significant improvement in macular thickness (-27 μm, p = 0.018). In contrast to our current study only 22% of patients in the TURF trial were dry at 6 months. However, a review of the baseline characteristics of patients in the TURF trial reveals that 17% were dry at baseline and are thus are not representative of patients in either our current study or the other published data.
In line with the other published studies [8,28,29] we found that patients with recalcitrant nAMD experienced a rapid and significant reduction in both their CMT and macular volumes after switching to aflibercept. This reduction was maintained beyond 12 weeks when the treatment interval was extended out to 8 weekly. Furthermore 30/48 of our patients, who previously had persisting fluid despite numerous treatment with either or both ranibizumab or bevacizumb had no subretinal fluid at week 48. The 3 other prospective AFL switch studies have reported broadly similar data [8,28,29]. The fact that a greater proportion of patients in our study were dry at week 48 compared to week 24 is intriguing and not easily explained. However, a similar trend was observed in the other anatomical parameters that we measured. Furthermore, these findings are consistent with data reported by others who have studied this phenomenon [28] suggesting that the neovascular complexes were stabilizing on long term therapy. As was observed in the landmark VIEW studies both the CMT and the proportion of patients with persisting subretinal fluid worsened once the treatment interval was extended beyond 4 weeks in our cohort. Similar results were reported by Chang et al. [8]. Whilst it remains unproven that a drier macular equates to better visual outcomes the fact the fluid re-accumulates in some patients after 4 weeks suggests that the neovascular complex has been allowed to reactivate. As such our data lends weight to the call for clinicians to tailor treatment to the individual [31].
Increasingly it is being recognized that the subretinal component of the neo-vascular complex, and in particular the serous component of PEDs, may respond particularly well to intravitral aflibercept [5,9,10,12,24,26,28,29]. At present little attention is paid to how the different components of the neovascular complex respond to anti-VEGF treatment, with the current emphasis being placed on the relatively easily measured parameter of central macular thickness. However, when measuring both central macular thickness and macular volume the Heidleberg software tends to default to comparing the distance between the RPE/Bruchs membrane complex and the inner limiting membrane. Whilst such a computation will capture the resolution of sub- and intra-retinal fluid after treatment, any change in the sub RPE component of the neo-vascular complex will go unreported. We were particularly interested to record the change in the sub RPE component of the neovascular complex and thus devised a protocol, utilizing the manual adjustment of the segmental lines generated automatically by the Heidleberg software, to measure this accurately in a sub group of our patients. We found that where the neo-vascular complex had a sub RPE component, this sub RPE component reduced considerably after treatment with aflibercept with a significant reduction both in the PED height and volume . Of the 32 patients in our current study who had PEDs at baseline the PED improved in 18 and resolved completely in two patients. Of those patients whose PED did not respond to treatment all had solid PEDs with mixed or hyper reflective elements. It is increasingly recognised that the response of the PED to aflibercept is dependent upon the characteristics of the PED, with the exudative and haemorrhagic components of the PED being particularly responsive [10,13,32]. Our data adds further support to this observation. Similar improvements in the sub RPE component of the nAMD lesion after treatment with intravitreal aflibercept have now also been reported elsewhere [5,9,10,12,13,24,26,28,29]. These data lend support to the hypothesis that aflibercept may be more effective than either ranibizumab or bevacizumab in the treatment of neovascular complexes, particularly the PCV variant that contain a significant sub RPE component [29,33-35]. Further studies are however needed to test this hypothesis but recent data reporting the efficacy of aflibercept in the management of PCV appears to support this [29,36,37].
Despite a significant improvement in the anatomical parameters of the nAMD leison, patients in our study did not experience a significant improvement in their visual acuity or contrast sensitivity after switching treatments. Whilst some studies have reported visual gains after switching to agents [8,9,11] others have shown no improvement [5,10,12,26,28,29]. The lack of improvement in visual acuity in our current study is likely to be a reflection of the chronicity of the disease with its attendant loss of the photoreceptor matrix, as the mean duration of disease in our cohort was 35 months. If these patients had been switched to aflibercept earlier they may have achieved better functional outcomes but, as previoulsy acknowledged, the question of whether a drier macula achieves better long-term functional results remains unanswered [38].
In conclusion, our current study provides longer term outcome data on the effect of switching to aflibercept in patients who have shown an inadequate anatomical response to either bevacizumab or ranibizumab. Consistent with the published literature, we were able to show a statistically significant decrease in CMT at 12 weeks, and the beneficial effect was maintained at 48 weeks when patients were extended to an 8 weekly fixed treatment cycle. Furthermore, 62% of patients had resolution of what had previously been recalcitrant subretinal fluid, at the close of the study. One potential limitation of our study is the heterogeneity of the study population, in terms of the lesion types and initial anti-VEGF agent used. However, our reasonably large patient cohort with 1 year prospective follow-up data represents "real world' practice and thus has an important contribution to the management of these difficult to manage patients with recalcitrant disease. This study demonstrates that switching patients with recalcitrant nAMD to IVT-AFL led to statistically significant improvements in all the surrogate markers of lesions activity namely; CMT, macula volume, PED height and volume at 48 weeks. BCVA was maintained during the study period. No significant adverse events were noted.
Acknowledgments
The authours would like to acknowledge Mr Andrew Worsley and Mrs Hannah Binnie for statistical analysis. We would like to thank Mr Jason Dhana who was involved in refraction measurements. We would also like to acknowledge Dr Andrea Vincent who was involved in administering treatments to patients at Retina Specialists, Parnell, Auckland, New Zealand. This study was supported by Bayer New Zealand who supplied the study drug.
Ethics Statement
This trial has received ethics approval from New Zealand Health and Disability Ethics Committee (13/NTB/66). It was registered on the Australian New Zealand Clinical Trials Registry (ACTRN12613001020774).
References
-
IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, et al. (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119: 1399-1411.
-
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, et al. (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119: 1388-1398.
-
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, et al. (2012) Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degenration. Ophthalmology 119: 2537-2548.
-
Ehlken C, Jungmann S, Böhringer D, Agostini HT, Junker B, et al. (2014) Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye (Lond) 28: 538-545.
-
Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, et al. (2013) Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 156: 15-22.
-
Cho H, Shah CP, Weber M, Heier JS (2013) Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 97: 1032-1035.
-
Kumar N, Marsiglia M, Mrejen S, Fung AT-C, Slakter J, et al. (2013) Visual and Anatomical Outcomes of Intravitreal Aflibercept in Eyes with Persistent Subfoveal Fluid Despite Previous Treatments with Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration. Retina 33: 1605-1612.
-
Chang AA, Li H, Broadhead GK, Hong T, Schlub TE, et al. (2014) Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Ophthalmology 121: 188-192.
-
Fassnacht-Riederle H1, Becker M, Graf N, Michels S (2014) Effect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMD. Graefes Arch Clin Exp Ophthalmol 252: 1705-1709.
-
Gharbiya M, Iannetti L, Parisi F, De Vico U, Mungo ML, et al. (2014) Visual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Biomed Res Int 2014: 273754.
-
Michalewski J, Nawrocki J, Trębińska M, Michalewska Z (2014) Switch to a single dose of aflibercept in bevacizumab nonresponders with AMD. Can J Ophthalmol 49: 431-435.
-
Thorell MR, Nunes RP, Chen GW, Doshi RR, Dugar J, et al. (2014) Response to aflibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMD. Ophthalmic Surg Lasers Imaging Retina 45: 526-533.
-
Broadhead GK, Hong T, Zhu M, Li H, Schlub TE, et al. (2015) Response of Pigment Epithelial Detachments to Intravitreal Aflibercept Among Patients With Treatment-Resistant Neovascular Age-Related Macular Degeneration. Retina 35: 975-981.
-
Ho AC, Busbee B, Regillo CD, Wieland MR, VanEveren SA, L, et al. (2013) Twenty-four-Month Efficacy and Safety of 0.5mg or 2.0mg Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration. Ophthalmology 121: 2181-2192.
-
Brown DM, Chen E, Mariani A, Major JC (2013) Save Study Group. Super-dose Anti-VEGF (SAVE) Trial: 2.0mg Intravitreal Ranibizumab for Recalcitrant Neovascular Macular Degeneration - Primary End Point. Ophthalmology 120: 349-354.
-
Wykoff CC, Brown DM, Croft DE, Wong TP (2013) Two Year SAVE Outcomes: 2.0 mg ranibizumab for recalcitrant neovascular AMD. Ophthalmology 120: 1945-1946.
-
Fung AT, Kumar N, Vance SK, Slakter JS, Klancik JM, et al. (2012) Pilot study to evaluate the role of high-dose rAnibizumab 2.0mg in the management of enovascular age-related macular degeneration in patients with perSistent/recurrenT macular fluid <30 days following treatment with intravitreal anti-VEGF therapy (the LAST Study). Eye 26: 1181-1187.
-
Moisseiev E, Katz G, Moisseiev J, Loewenstein A, Goldstein M, et al. (2015) Switching Treatment for Neovascular Age-Related Macular Degeneration from Bevacizumab to Ranibizumab: Who is Likely to Benefit From the Switch? Retina 35: 1323-1330.
-
Stewart MW, Grippon S, Kirkpatrick P (2012) Aflibercept. Nat Rev Drug Discov 11: 269-270.
-
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, et al. (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 99: 11393-11398.
-
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, et al. (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15: 171-185.
-
Stewart MW, Rosenfeld PJ (2008) Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 92: 667-668.
-
Yung WK (2008) Moving toward the next steps in angiogenesis therapy? Neuro Oncol 10: 939.
-
Patel KH, Chow C, Rathod R, Mieler WF, Lim JI, et al. (2013) Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye 27: 663-668.
-
Heussen FM, Shao Q, Ouyang Y, Joussen AM, Müller B (2014) Clinical outcomes after switching treatment from intravitreal ranibizumab to aflibercept in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 252: 909-915.
-
Batioglu F, Demirel S, Özmert E, Abdullayev A, Bilici S (2015) Short-term outcomes of switching anti-VEGF agents in eyes with treatment-resistant wet AMD. BMC Ophthalmol 15: 40.
-
Wykoff CC, Brown DM, Chen E, Major JC, Croft DE, et al. (2013) SAVE (Super-dose anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surg Lasers Imaging Retina 44: 121-126.
-
Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG (2014) Visual and anatomic outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye 28: 895-899.
-
Kawashima Y, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, et al. (2015) Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 253: 1471-1477.
-
Wykoff CC BD, Maldonado ME, Croft DE (20104) Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol 98: 951-955.
-
Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, et al. (2015) TREAT-AND-EXTEND REGIMENS WITH ANTI-VEGF AGENTS IN RETINAL DISEASES: A Literature Review and Consensus Recommendations. Retina 35: 1489-1506.
-
Punjabi OS, Huang J, Rodriguez L, Lyon AT, Jampol LM, et al. (2013) Imaging characteristics of neovascular pigment epithelial detachments and their response to anti-vascular endothelial growth factor therapy. Br J Ophthalmol 97: 1024-1031.
-
Miura M, Iwasaki T, Goto H (2013) Intravitreal aflibercept for polypoidal choroidal vasculopathy after developing ranibizumab tachyphylaxis. Clin Ophthalmol 7: 1591-1595.
-
Yonekawa Y (2013) Aflibercept for the treatment of refractory polypoidal choroidal vasculopathy. Can J Ophthalmol 48: e59-60.
-
Yamashita M, Nishi T, Hasegawa T, Ogata N (2014) Response of serous retinal pigment epithelial detachments to intravitreal aflibercept in polypoidal choroidal vasculopathy refractory to ranibizumab. Clin Ophthalmol 8: 343-346.
-
Ijiri S, Sugiyama K (2015) Short-term efficacy of intravitreal aflibercept for patients with treatment-naive polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 253: 351-357.
-
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, et al. (2015) One-Year Results of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy. Ophthalmology 122: 1866-1872.
-
Gianniou C, Dirani A, Jang L, Mantel I (2015) refractory intraretinal or subretinal fluid in neovascular age-related macular degeneration treated with intravitreal ranizubimab: Functional and Structural Outcome. Retina 35: 1195-1201.





