Journal of Dermatology Research and Therapy
Sun Sensitivity and Sunburns as Related to Cutaneous Melanoma among Populations of Spanish Descent: A Meta-Analysis
Leslie K Dennis1,2*, Stephanie G Lashway1 and Marvin E Langston1
1Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ, USA
2Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, IA, USA
*Corresponding author:
Leslie K Dennis, Department of Epidemiology & Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, 1295 N Martin Ave, Tucson, AZ 85724, P.O. Box 24521, USA, E-mail: ldennis@email.arizona.edu
J Dermatol Res Ther, JDRT-1-009, (Volume 1, Issue 1), Research Article; ISSN: 2469-5750
Received: October 8, 2015 | Accepted: November 14, 2015 | Published: November 16, 2015
Citation: Dennis LK, Lashway SG, Langston ME (2015) Sun Sensitivity and Sunburns as Related to Cutaneous Melanoma among Populations of Spanish Descent: A Meta-Analysis. J Dermatol Res Ther 1:009. 10.23937/2469-5750/1510009
Copyright: © 2015 Dennis LK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Few studies have examined sun sensitivity risk factors for cutaneous melanoma specifically in populations of Spanish descent. Previous searches were conducted in PUBMED for articles on melanoma and sun exposure through 2008. Over 300 articles were reviewed and relevant data was abstracted. These abstract forms were subsequently reviewed for studies in populations of Spanish descent. PUBMED was then examined for more recent studies of melanoma in populations of Spanish descent. Eight appropriate articles were found, which comprised 7 discrete studies. We conducted a meta-analysis of these seven studies analyzing Fitzpatrick skin type, skin color and history of sunburns. The risk of melanoma was increased for fair vs. dark skin color (OR = 2.9, 95% CI 2.0-4.1) and for skin type I & II vs. II I& IV (OR = 3.5, 95% CI of 2.0-6.1). However, when skin type was examined as an ordered categorical factor in a linear dose-response analysis, a 12-fold difference was seen between skin type I and IV. Any history of sunburn in childhood and lifetime were also associated with melanoma with ORs of 5.6 and 4.0, respectively. The magnitudes of associations seen in this population were much higher than seen in previous meta-analyses of all studies of melanoma. These results provide some evidence of discrepancies of reporting skin color in heterogeneous populations including those of Spanish descent. Future studies should provide a more accurate measure of self-reported skin color in these populations.
Keywords
Hispanic, Melanoma, Sun sensitivity, Sunburn
Abbreviations
CI: Confidence interval, OR: Odds ratio, US: United States
Introduction
The incidence of cutaneous melanoma is increasing in the United States (US), with an average annual increase of 2.3% between 1999 and 2008 [1]. Other countries have also reported substantial increases [2-5]. Most studies of melanoma are restricted to European populations as cutaneous melanoma incidence is highest in these groups and lowest in African populations [6]. Within European populations, differences in incidence rates are attributed to variation in pigment or skin color between northern European and Latin populations [2,7,8]. Incidence rates and epidemiological studies have shown that individuals with fair complexions are at highest risk. Cutaneous melanoma has been strongly associated with sun sensitivity factors [9,10] and intermittent sun exposure [9] including history of sunburns [11]. However, less is known about how such factors vary across European populations, more specifically in populations of Spanish descent.
This study looked at the magnitude of association between sun sensitivity factors and cutaneous melanoma in populations of Spanish descent. Spanish descent, sometimes referred to as Hispanic, is defined as individuals who trace their ancestry back to a Spanish speaking country. This does not include other "Latin" populations such as those of Portuguese descent. Only a few studies have looked at these risk factors and melanoma in Hispanic populations. This meta-analysis pools sun sensitivity factors and sunburns as related to melanoma among these populations.
Methods
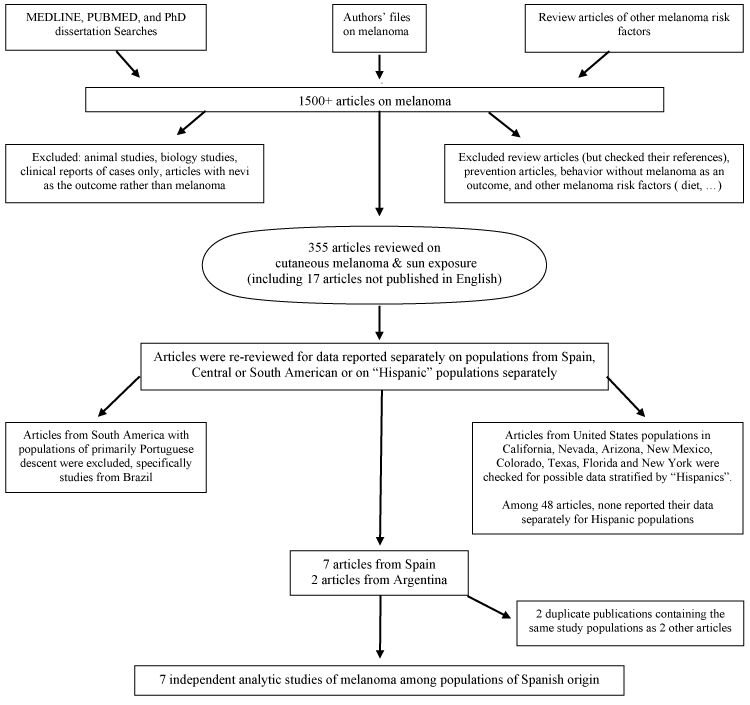
Analytic studies of cutaneous melanoma reporting on Hispanic populations were eligible for this meta-analysis. A search was conducted in Medline, dissertation databases, and PUBMED for key words related to melanoma and "sun", including sun, sunlight, tanbed, sunbed, artificial UV and sunburns, to obtain articles on any aspect of sun sensitivity, sun exposure or sunburns and risk of cutaneous melanoma. We repeatedly searched the PubMed database through December 2014. The titles and abstracts of over 1,500 articles were screened to exclude case reports, commentaries or editorials, animal studies, therapies, biological aspects of melanoma, and other irrelevant articles (Figure 1). Articles were gathered and their references checked for additional relevant studies. Data were abstracted from 355 articles reviewed in-depth.
The studies considered in this meta-analysis included cross-sectional studies, case-control studies, and cohort studies that reported populations of Spanish descent separately. We defined such populations as those in Spain; Central and South American countries colonized by Spain and self-classified Hispanics in the US. Initially all populations from Spain and South America were considered candidate populations; however, several studies among Brazilians were excluded as historically those populations identify themselves as Portuguese and not as Spanish. A multi-national study that included Spain but only reported it combined with data from Italy, France and Portugal was also excluded [12]. Several US studies where populations were likely to include Hispanics (Texas, California, Arizona, Nevada, Colorado and New York), were reviewed, however, none of these 48 articles stratified by ethnicity. The final study sample included studies among populations in Spain and Argentina. Non-English-language articles were reviewed by an experienced researcher fluent in the appropriate language to determine the relevance and, if needed, they completed an abstraction form. Translated information on the Argentine study [13] published in Spanish was used. After the review of the populations, 7 independent studies were identified (Figure 1).
Case-control studies were classified as population-based, hospital based, or in-between depending on how the control group was recruited. Population-based case-control studies appear to recruit controls from the general public. Among these studies the methods of recruitment include random selection of participants in health promotion program. Hospital based case-control studies include those with controls from a hospital, clinic, emergency room, or dermatology office. In-between studies include those where the controls were recruited from hospital visitors, non-relative visitors to dermatology departments or wards, or Madrid College of Lawyers. We combined scores of 4 different classification systems to assess the quality of included studies. The first 5-point quality assessment scale included items based on classification of controls (population-based, in-between, or hospital based), classification of cases (reporting incident melanomas, reporting if in-situ and/or invasive) and random selection of controls. Three other 5-points scales, for a total of 20 points, included: 1) case description, 2) control description (describing how recruited, reasons for eligibility, and numbers at stages of recruitment), and 3) adequate description of how potential confounders were identified and how they were chosen (low score indicated absence of defined criteria or moderately low for using p-values to determine confounding). Total points for the quality assessment are shown in Table 1. Weighting estimates based on the quality assessment greatly added to the heterogeneity, thus such estimates are not reported.
![]()
Table 1: Study populations included in this meta-analysis of sun sensitivity, sunburns and melanoma among populations of Spanish descent.
View Table 1
For each study and level of exposure, the natural log of the odds ratio (ln (OR)) and its variance are required for analyses. When available, the variances were calculated on the basis of the reported confidence intervals (CIs) from the most adjusted models reported (thus accounting for the adjustment). For studies not reporting ORs and CIs, we could only estimate crude ORs and their variances based on the number of exposed and unexposed with and without melanoma. Data were pooled by specific risk factors with three or more studies providing similar categorical data. The statistical analyses were performed using Microsoft Excel and SAS software. We report both the crude estimates from all studies and the pooled estimates of the most adjusted estimates reported for each study.
ORs were pooled using a weighted least squares method assuming a fixed effects model, a random effects model or as a linear dose-response [14-16]. ORs for dichotomous factors were pooled using fixed effects and random effects models [14,15]. The fixed effects method for pooling data is a simple model to understand and equivalent to pooling across strata (such as age), such that ln(OR)p = (∑ ln(ORi)/var(lnORi))/(∑ 1/var(lnORi), where p is the pooled estimate and i represents the individual studies being pooled. Thus, it is a variance weighted average. The pooled variance is the sum of the inverse variances such that Var(ln(OR)p = (∑ 1/var(lnORi))-1. Random effects models are more conservative than fixed effects models since they are assuming the risks are random across studies and the fixed effects models assume the studies are homogeneous [14,15].
For ordinal categories, a fixed-effects method was used to evaluate possible dose-response relationships in the ln(OR) as a linear function [16]. Greenland and Longnecker (1992) recommend the use of median scores to weight each exposure category, but few articles report medians or means; thus midpoints of reported ranges were used. To conduct a meaningful dose-response analysis or other sub-analyses, at least 3 studies must report the information needed.
Heterogeneity was estimated using the between-study variance (chi-square test for heterogeneity, Q), but interpretation is limited [17]. For the H statistic the ratio of Q to n-1 measures the extent of heterogeneity. The I2 statistic, [I2 = (H2-1)/H2], measures the amount of total variability in the risk estimates that is due to heterogeneity between studies [17]. Each of these statistics uses different assumptions to estimate the impact of heterogeneity, thus we examined them all. The percentage of total variability (I2) in risk estimates that is due to heterogeneity between studies was reported [17].
Results
Seven distinctive studies of melanoma in populations of Spanish descent were found from 8 articles [13,18-24] reporting association between sun sensitivity and melanoma. Two studies reported among Argentines [18-20] and five studies reported on subjects in Spain [13,21,22,24]. All studies were case-control studies. Most studies mentioned matching controls to cases on age and sex, but were unclear if such matching was one-to-one or frequency matching. They appeared to be frequency matching based on the ratio of cases to controls and types of analyses run. The studies pooled are described in Table 1 along with their report quality scores.
Among the various measures of sun sensitivity and sun exposure these studies reported, several studies reported skin color (fair vs. dark), skin type (I & II vs. III& IV), and ever having a sunburn during childhood or lifetime (Table 2). The risk of melanoma was higher for fair complexion measured using skin type (OR = 3.5, 95% CI of 2.0-6.1) than skin color (OR = 2.9, 95% CI 2.0-4.1). While ever compared to never having a sunburn (during a specific time period) are rough measures, they are strong indicators of melanoma risk with an OR = 5.6 (95% CI 3.0-10.3) for childhood sunburns and 4.0 (95% CI 1.6-9.9) for lifetime sunburns (Table 2). Not all reported ORs in the studies were able to be pooled when studies did not report similar categories, such as hair color, eye color, and adolescent sunburns. For those factors that were pooled, there was a great amount of inter-study variability based on I2 ranging from 47% to 99% (Table 2).
![]()
Table 2: A meta-analysis pooling odds ratios for cutaneous melanoma and dichotomous factors across case-control studies in populations of Spanish descent based on a random effects model.
View Table 2
We were able to further quantify skin type and lifetime sunburns through linear dose-response analyses as three studies reported categories of these factors. The dose response analyses are linear in the beta or ln(OR). For skin type, an OR of 2.3 (95% CI 2.0-2.7) was observed for the difference between any two consecutive skin types (I, II, III, or IV). This can also be interpreted as an OR of 12.3 (95% CI 7.4-20.5) when comparing skin type I to skin type IV (Table 3). However, even among only these three studies, heterogeneity was large (70.4%). Similarly, an increase in melanoma risk was seen for each increasing number of sunburns between two individuals for an OR of 1.6 (95% CI 1.4-1.9) for an increase of 5 sunburns, but again with large heterogeneity (79.3%).
![]()
Table 3: A meta-analysis pooling odds ratios for cutaneous melanoma and dose information for continuous or ordered categorical factors across case-control studies in populations of Spanish descent based on a random effects model.
View Table 3
Discussion
These studies in populations of Spanish descent had stronger associations (higher effect sizes) for skin type I compared to type IV, OR = 12.4, than did all melanoma studies reporting type I compared to type IV (OR = 2.1) by Gandini et al. [10]. Skin color can be compared more directly with an OR of 2.9 for light vs. dark skin color among populations of Spanish descent to OR of 2.1 for light vs. dark skin color among all studies [10]. The differences in magnitude of these ORs may represent different distributions of skin color and skin type, different interpretations of skin color or aspects of skin type or overall measurement issues.
Sun sensitivity factors attempt to quantify fair complexion. Fair complexion is measured using a variety of sun sensitivity factors including Fitzpatrick skin type (determined by a clinician), self-reported skin type, tendency to sunburn on first exposure to the sun in the spring/summer, inability to tan after prolonged and repeated sun exposure, eye color, hair color and skin color. However, skin color in White populations (including those of northern European descent and Spanish descent) appears to require a more complex scale than the typically used "fair", "medium" or "dark" skin. When describing skin color among populations of Spanish descent, it is unclear if such subjects would represent the same range of skin color variation with a different distribution within the range, or if such populations may include darker gradients of complexion, resulting in lower rates of cutaneous melanoma.
To look at this further, Table 4 outlined how these risk factors were defined within each study compared to a generalization among most melanoma studies among White non-Hispanic populations. Hair and eye color appears to be asked using fairly standard questions and responses among studies. Expectantly, skin type is also reported with consistent categories since it is based on clearly defined Fitzpatrick skin type [25]. However, for several of the Spanish studies, medium skin color does not appear to be an option. This could then change how participants interpret fair and dark skin categories. Such categories may be less informative among populations of Spanish descent and may be interpreted differently. Reported associations between skin color and melanoma may be underestimated in studies that do not consider Spanish descent as seen with over half of the subjects in these seven studies reporting they have fair skin. Tendency to sunburn and inability to tan were not asked in these populations of Spanish descent. While we know that skin type is defined based on tendency to sunburn and inability to tan, some studies have shown inconsistent translation of tendency to sunburn and inability to tan to a separate question on skin type by subjects when asked as to report all three measures. More research in the area of how sun sensitivity may vary by populations is needed.
![]()
Table 4: Study populations included in this meta-analysis of sun sensitivity, sun burns and melanoma among populations of Spanish descent.
View Table 4
Simplified comparisons of ever versus never experiencing a sunburn during childhood or over a lifetime found a higher association with childhood (OR = 5.6) compared to lifetime (OR = 4.0). The higher effect size (OR) for childhood than lifetime sunburns is consistent with what was seen among all studies of melanoma [11] with ORs of 1.9 and 1.6, respectively. However, the ORs among populations of Spanish decent show a greater effect of sunburn on risk of melanoma. To understand why this may be, we would need to look at data on number of sunburns within different time periods over the life course. Unfortunately, these studies only reported enough data to pool across lifetime number of sunburns. These data showed an OR of 1.6 for an increase of 5 sunburns over a lifetime with a large variation between studies. The similar pooled risk among all melanoma studies was 1.22 (95% CI of 1.18-1.26) for an increase of 5 sunburns during a lifetime [11]. The stronger association seen among populations of Spanish descent may reflect fewer Spanish subjects with sunburns and such sunburns may represent not only sun exposure but fairer skin which has put them at higher risk of melanoma. It may also reflect lack of proper adjustment for sun sensitivity, which is a large confounder of the association between melanoma and sunburns [11].
Studies did not consistently report how they defined sunburns to subjects. Two of the seven studies did define sunburns as blistering (thus more notable), whereas the other studies just mentioned "sunburns". The lack of definition for sunburns is a consistent issue among all melanoma studies of sunburns [11]. Therefore, heterogeneity between studies could be explained somewhat by differing definitions of sunburns from any mild sunburn to one so severe it caused blistering (or pain lasting 2 or more days). Additional heterogeneity could arise from differing definitions of childhood sunburns from ages 0-15, < age 18, or unstated definitions. These are reporting issues that plague all of the melanoma literature making exposure assessment difficult.
Future studies of melanoma risk should always consider adjustment for confounding from sun sensitivity factors. They should also attempt to clarify or create reliable and valid instruments to define self-reported skin color with more variation that can be used among both Hispanic and non-Hispanic Whites and quantify differences in skin color among different populations. To do so, concepts of tendency to sunburn and inability to tan may need to be included or asked separately to questions on skin color and/or skin type.
References
-
Simard EP, Ward EM, Siegel R, Jemal A (2012) Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 62: 118-128.
-
Osterlind A (1992) Epidemiology on malignant melanoma in Europe. Acta Oncol 31: 903-908.
-
Gallagher RP, Ma B, McLean DI, Yang CP, Ho V, et al. (1990) Trends in basal cell carcinoma, squamous cell carcinoma, and melanoma of the skin from 1973 through 1987. J Am Acad Dermatol 23: 413-421.
-
Hoey SE, Devereux CE, Murray L, Catney D, Gavin A, et al. (2007) Skin cancer trends in Northern Ireland and consequences for provision of dermatology services. Br J Dermatol 156: 1301-1307.
-
Wallingford SC, Alston RD, Birch JM, Green AC (2011) Increases in invasive melanoma in England, 1979-2006, by anatomical site. Br J Dermatol 165: 859-864.
-
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-386.
-
Jensen OM, Esteve J, Møller H, Renard H (1990) Cancer in the European Community and its member states. Eur J Cancer 26: 1167-1256.
-
Forsea AM, Del Marmol V, de Vries E, Bailey EE, Geller AC (2012) Melanoma incidence and mortality in Europe: new estimates, persistent disparities. Br J Dermatol 167: 1124-1130.
-
Armstrong BK, Kricker A (2001) The epidemiology of UV induced skin cancer. J Photochem Photobiol B 63: 8-18.
-
Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, et al. (2005) Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer 41: 2040-2059.
-
Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, et al. (2008) Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Ann Epidemiol 18: 614-627.
-
Zanetti R, Rosso S, Martinez C, Nieto A, Miranda A, et al. (2006) Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer 94: 743-751.
-
Ruiz Lascano A, Kuznitzky R, Cuestas E, Mainardi C, Albertini R, et al. (2004) Risk factors for cutaneous melanoma: case-control study in Cordoba, Argentina. Medicina (B Aires) 64: 504-508.
-
Woolf B (1955) On estimating the relation between blood group and disease. Ann Hum Genet 19: 251-253.
-
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177-188.
-
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135: 1301-1309.
-
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539-1558.
-
Loria D, Matos E (2001) Risk factors for cutaneous melanoma: a case-control study in Argentina. Int J Dermatol 40: 108-114.
-
Fernandez L, Milne R, Bravo J, Lopez J, Aviles J, et al. (2007) MC1R: three novel variants identified in a malignant melanoma association study in the Spanish population. Carcinogenesis 28: 1659-1664.
-
Fernandez LP, Milne RL, Pita G, Floristan U, Sendagorta E, et al. (2009) Pigmentation-related genes and their implication in malignant melanoma susceptibility. Exp Dermatol 18: 634-642.
-
Espinosa Arranz J, Sanchez Hernandez JJ, Bravo Fernandez P, Gonzalez-Baron M, Zamora Auñon P, et al. (1999) Cutaneous malignant melanoma and sun exposure in Spain. Melanoma Res 9: 199-205.
-
Rodenas JM, Delgado-Rodriguez M, Herranz MT, Tercedor J, Serrano S (1996) Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control 7: 275-283.
-
Nagore E, Hueso L, Botella-Estrada R, Alfaro-Rubio A, Serna I, et al. (2010) Smoking, sun exposure, number of nevi and previous neoplasias are risk factors for melanoma in older patients (60 years and over). J Eur Acad Dermatol Venereol 24: 50-57.
-
Ballester I, Oliver V, Bañuls J, Moragon M, Valcuende F, et al. (2012) Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain. Actas Dermosifiliogr 103: 790-797.
-
Fitzpatrick TB (1988) The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 124: 869-871.