International Journal of Diabetes and Clinical Research
Ethanolic Extract of Bauhinia forficata: Metabolic Effects in Diabetic and Normoglycemic Rats
Liana de Oliveira Gomes1, Francislaine Aparecida dos Reis Lívero1, Aline Maria Stolf1, Carlos Eduardo Alves de Souza1, Michele Pontes Werneck1, Cristiane Vizioli de Castro Ghizoni1, Maria Helena Verdan1, José Ederaldo Queiroz Telles1, Rosangela Locatelli Dittrich1, Maria Élida Alves Stefanello1, Jurandir Fernando Comar1 and Alexandra Acco1*
1Department of Pharmacology, Federal University of Paraná, Curitiba, Brazil
2Department of Biochemistry, University of Maringá, Maringá, Brazil
3Department of Chemistry, Federal University of Paraná, Curitiba, Brazil
4Department of Medical Pathology, Federal University of Paraná, Curitiba, Brazil
5Department of Veterinary Medicine, Federal University of Paraná, Curitiba, Brazil
*Corresponding author:
Alexandra Acco, Department of Pharmacology, Federal University of Paraná (UFPR), Biological Science Sector, Centro Politécnico, CX P 19031, Curitiba -PR, Zip Code: 81531-980, Brazil, Phone: +55 (41) 3361-1742, Fax: +55 (41) 3266-2042, E-mail: aleacco@ufpr.br
Int J Diabetes Clin Res, IJDCR-3-061, (Volume 3, Issue 2), Original Article; ISSN: 2377-3634
Received: March 12, 2016 | Accepted: June 02, 2016 | Published: June 06, 2016
Citation: Gomes LO, Lívero FAR, Stolf AM, de Souza CEA, Werneck MP, et al. (2016) Ethanolic Extract of Bauhinia forficata: Metabolic Effects in Diabetic and Normoglycemic Rats. Int J Diabetes Clin Res 3:061. 10.23937/2377-3634/1410061
Copyright: © 2016 Gomes LO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Bauhinia forficata (BF) is used in folk medicine to reduce glycemia in diabetes. We evaluated the glucose hepatic metabolism (ex vivo) and the hypoglycemic effects (in vivo) of an ethanolic extract of BF leaves (BFEE) in normoglycemic and streptozotocin (STZ)-induced diabetic rats.
Methods: The livers of rats were perfused with BFEE (40 mg L-1) and glucose metabolism under fed or fasting conditions (L-glutamine as the substrate) was analyzed. Additionally, rats were divided into 4 groups for oral treatment: vehicle-treated non-diabetic rats; BFEE-treated (300 mg kg-1) non-diabetic rats; vehicle-treated diabetic rats; and BFEE-treated (300 mg kg-1) diabetic rats. Glycemia was measured acutely and after 7 days of treatment; plasma biochemistry, hepatic glycogen contents, and hepatic and pancreatic histology were evaluated after this period.
Results: Liver infusion of BFEE in fed and fasted diabetic rats reduced glucose and lactate production, respectively; however, BFEE did not affect glycemia or hepatic glycogen levels. Plasma transaminases increased in diabetic animals receiving the extract. The STZ model reduced by nearly half the number of Langerhans' islets in comparison to non-diabetic animals, however no pancreatic difference was observed between diabetic animals treated with extract and diabetic animals treated with vehicle.
Conclusions: BFEE interferes with hepatic glycolysis, glucogenesis, and lactate production from L-glutamine. Thus the BFEE effects depend of the alimentary state. However, it did not suppress hyperglycemia in diabetic rats.
Keywords
Bauhinia forficata, Experimental diabetes, Liver perfusion, Hepatic metabolism, Glucose, L-Glutamine
Introduction
Diabetes is a group of metabolic diseases characterized by hyperglycemia and associated complications, such as dysfunction and failure of the eyes, kidneys, nerves, brain, heart, and blood vessels. Diabetes may result from defects in secretion and/or insulin action involving specific pathogenic processes, including destruction of pancreatic beta cells, insulin resistance, and insulin secretion disorders [1]. The estimated prevalence of diabetes nowadays is about 150 million people worldwide [2], and this is expected to rise to 552 million by 2030 [3], increasing in numbers as much the adult population grows [4].
The most common form of diabetes is type 2 (T2DM), known as adult-onset diabetes and related with metabolic syndrome, followed by type 1 (T1DM), formerly known as juvenile diabetes and characterized by β-cell destruction which leads to absolute insulin deficiency [5]. T1DM is treated with insulin, while T2DM is treated with oral hypoglycemic agents, and exercise and diet as ancillary measures to control the disease. More than 400 plant species have been reported to possess hypoglycemic activity, but only a few have been investigated [2,5]. Among the plants commonly used to treat diabetes is the genus Bauhinia, particularly B. forficata (BF), known as "cow's foot", which is used as a tea in folk medicine. Phytochemical analysis confirmed the presence of flavonoids in the leaves of BF, highlighting kaempferitrin as the most active hypoglycemic compound [6-8].
Although studies have shown that 30-day administration of a decoction of BF leaves reduces plasma glucose in diabetic animals [9], some researchers performed studies with extracts obtained from the leaves of BF, to concentrate active compounds such as kaempferitrin [10-12]. Silva et al. [11] showed that the n-butanolic extract of BF reduces acute blood glucose in alloxan-induced diabetic rats, while Jorge et al. [13] suggest kaempferitrin functions as an insulin-mimic in diabetic rats. Tzeng et al. [14]. demonstrated that kaempferitrin promotes activation of the classical insulin pathway to activate GLUT4 translocation and stimulate continuous adiponectin secretion, promoting peripheral sensitivity to insulin. However, other authors have not observed hypoglycemic activity in BF preparations [15,16]. Higher doses of BF aqueous extract (500-1000 mg kg-1) in female rats had no hypoglycemic effect and did not improve maternal outcomes in diabetic animals [16].
Diabetes is associated with changes of the glucose metabolism also in the liver, such as increases in the hepatic glucose release and stimulus in the liver gluconeogenesis [17]. However, none studies have evaluated the effects of BF on hepatic metabolism of glucose in diabetic condition. In this context, there are many aspects of BF that need to be clarified. In the absence of consensus regarding BF bioactivities, we aimed to study the metabolic and hypoglycemic effects of ethanolic extract of BF leaves in streptozotocin-induced diabetic rats. To our knowledge, this is the first report to investigate the influence of BF in the metabolism of glucose into the liver.
Materials and Methods
Plant material, extraction and chemical analyses
Leaves of Bauhinia forficata Link was collected in the Curitiba City, Parana State, Brazil (25°26'34.85''S, 49°14'22.58'' W) in May/2012. A voucher specimen was deposited at the Herbarium of Museu Botânico Municipal de Curitiba (# MBM 384637). The leaves were dried under controlled temperature (40°C), powdered, and extracted with hexane, dichloromethane, ethyl acetate, and ethanol, successively. The solvents were removed under reduce pressure to give the respective extracts. Only Bauhinia forficata ethanolic extract (BFEE, yielding 2.5%) was used in the experiments. HPLC fingerprints of BFEE were recorded on a Waters HPLC equipped with a 2998 photodiode array detector, and a Nucleosil 100-5 C18 column (250 × 4.6 mm, 5 μm particle size). It was used the method proposed by Ferreres et al. [7] with modifications. The mobile phase consisted of H2O with 1% of acetic acid (A) and methanol (B) applied in a linear gradient from 80:20 (A:B) to 50% over 30 min, followed by 90% (B) at 35 min. After was kept isocratic for 5 min, and was applied a new gradient reaching 100% (B) at 50 min. The flow rate was 1 mL min-1, and the effluent was monitored at 340 nm.
Induction of experimental diabetes
Adult male Wistar rats, weighing between 180 and 200 g, were kept in a temperature-controlled room (20-23°C) and fed ad libitum with a balanced commercial chow and water. Diabetes was induced by intraperitoneal administration of 50 mg kg-1 of streptozotocin (STZ; Santa Cruz Biotechnology, K2911) diluted in citrate buffer 10 mM (pH 4.5) after fasting for 12 h [18]. Administration of this toxin produces a diabetic status compatible with type I diabetes; disease status was confirmed by measuring glycemia (via tail puncture) with reactive strips in a glucometer (Accu-Chek®, A F Hoffmann-La Roche AG, Basel, CH). The animals were considered diabetic when the glycemia was > 250 mg dL-1. Experiments or treatments were initiated 7 days after STZ administration, a period necessary for disease stabilization.
Ex vivo study: Liver perfusion
The effects of BFEE in hepatic glucose metabolism were evaluated in isolated perfused livers in an ex vivo method. For the surgical procedure, rats were anesthetized by intra peritoneal injection of ketamine (100 mg kg-1) and xylazine (10 mg kg-1). Hemoglobin-free and non-recirculating perfusion was performed as described by Bracht et al. [19]. After cannulation of the portal and cava veins, the liver was positioned in a plexiglass chamber. Flow was maintained by a peristaltic pump (Minipuls 3, Gilson, France) between 28 and 35 mL min-1, depending on liver weight. The perfusion fluid was Krebs/Henseleit-bicarbonate (KH) buffer (pH 7.4) containing 0.025% bovine serum albumin, saturated with a mixture of oxygen and carbon dioxide (95:5) by means of a membrane oxygenator with simultaneous temperature adjustment (37°C). Substrates and BFEE were added to the perfusion fluid according to the following protocols applied to diabetic and normoglycemic animals.
Effects of BFEE on liver glycogen catabolism and glycolysis: Evaluated in fed rats (n = 3), whose livers were perfused with KH for 50 min, with BFEE infusion (40 mg L-1) from the 10th to the 40th minute.
Effects of BFEE on liver gluconeogenesis: Evaluated in 16-h fasted rats (n = 3-4), whose livers were perfused with substrates L-glutamine (2.5 mM) and ammonium chloride (0.6 mM) from the 10th to the 82nd minute of perfusion, and BFEE (40 mg L-1) concurrently from the 50th minute.
Samples of the effluent perfusion fluid were collected every 2 or 4 min and analyzed by standard enzymatic procedures for glucose, lactate, and pyruvate [20]. The oxygen concentration in the outflowing perfusate was monitored continuously with a Teflon-shielded platinum electrode positioned in the plexiglass chamber at the exit of the perfusate [19]. Metabolic rates were calculated from input-output differences in total flow rate and were referred to the wet weight of the liver.
In vivo study: Rat groups, treatment schedule, and sample collection
To check the in vivo hypoglycemic effects of BFEE, an experiment was performed in normoglycemic (control) and diabetic rats. The treatment started 7 days after STZ administration and was conducted for the next 7 days, with 4 groups of animals (n = 6): [1] diabetic rats treated with extract, [2] diabetic rats treated with vehicle (tween 80 plus water), [3] normoglycemic rats treated with extract, and [4] normoglycemic rats treated with vehicle. Preliminary experiments with a dose of 150 mg kg-1 BFEE in diabetic rats for 7 days produced no hypoglycemic effect (data not shown); thus, we chose an orally administered dose of 300 mg kg-1 BFEE. For some experiments a positive control group [5] was used, composed by diabetic rats treated subcutaneously with 6.0 IU NPH insulin, once a day. The variation in the rats' body weight was also measured during the treatment.
At the end of the treatment period, animals were anesthetized with intraperitoneal ketamine (100 mg kg-1) and xylazine (10 mg kg-1) and sample materials were collected (blood, liver, and pancreas). Blood was collected by cava vein puncture; the plasma was separated by centrifugation and kept at -20°C. After harvest, fragments of the liver and pancreas were immediately placed in 4% buffered formalin for histological studies, and part of the liver was stored at -80°C. The animals were euthanized by diaphragmatic puncture.
Evaluation of the hypoglycemic potential of the BFEE: Two experiments were performed in each animal subsequently to evaluate the effects of BFEE on blood glucose: [a] Acute, in which blood glucose levels were measured before and 1 and 3h after administration of the extract or vehicle; and [b] Subacute, after 7 days of treatment with extract or vehicle. In both experiments, glycemia was measured in tail vein blood with reactive strips in a glucometer.
Plasma biochemistry: Hepatocyte integrity and blood protein glycosylation were assessed through plasmatic parameters. Measurements of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and fructosamine were performed using commercial kits (Kovalente, São Gonçalo -RJ - Brazil) in an automated system (Mindray BS-200, Shenzhen - China).
Determination of hepatic glycogen content: The determination of liver glycogen was performed as described by Kepler & Decker [21], starting from 2.0 g of frozen tissue. The liver was minced with liquid nitrogen in a pestle and a 5× volume of 0.6 N perchloric acid was added to macerate the sample. The contents were transferred to a tube and homogenized in a Potter-elvehjem homogenizer. The glucose content of the homogenate was measured and named "basal glucose". The hydrolysis of glycogen in this homogenate was achieved with the addition of 1.0 M potassium bicarbonate, 0.2 M amyloglucosidase, and acetate buffer, pH 4.8. The pH of the mixture was adjusted to 6.0. The tube was covered and placed in a 40°C water bath and stirred for 2 h. The reaction was stopped by addition of 0.6 N perchloric acid and the samples were centrifuged at 6000 rpm for 5 min at 4°C. Total glucose in the supernatant ("final glucose") was determined with a commercial kit and a spectrophotometer at 505 nm. The difference between basal and final glucose was considered the glycogen content and expressed as glycosyl units.
Histology of the pancreas and liver: To complement the previous experiments and to observe any changes induced by diabetes or treatment with BFEE, samples of the pancreas and liver were collected for histology. After fixation, samples were dehydrated in a graded series of ethanol and xylene before paraffin embedding. Thin sections (4 μm) were processed for histology and stained with hematoxylin and eosin (HE) [22]. Groups were compared and lesions were scored (0-3) as follows: 0, no change; 1, mild changes; 2, moderate changes; and 3, pronounced changes. A blind histological evaluation was performed by a pathologist in all rats belonged to the groups 1, 2, 3 and 4 described above (In vivo study).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). The comparison between experimental groups was made by analysis of variance (ANOVA) with Bonferroni correction. Metabolites obtained in liver perfusion were assessed by nonparametric Student's t test. Glycemia was analyzed by two-way ANOVA, with time and treatment been the parameters. Differences were considered significant when p < 0.05. The analyses were performed using the statistical program GraphPad Prism version 5.0.
Results
Chemical analyses of the ethanolic extract of B. forficata (BFEE)
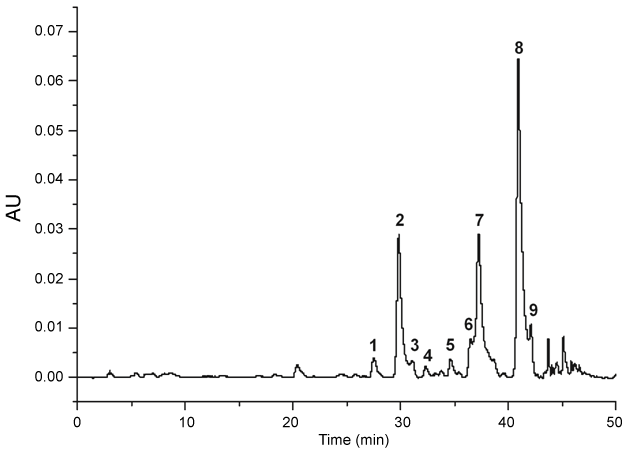
The HPLC profile of BFEE is showed in the (Figure 1). It were detected nine flavonoids, being two quercetin derivatives (1 and 4, UV λmax 256, 355 nm) and seven kaempferol derivatives [2,3,5-9], UV λmax 266, 348 nm). Peak 2 was identified as kaempferol-3,7-O-(α)-dirhamnoside (kaempferitrin) by comparison with literature [7].
.
Figure 1: HPLC-DAD (340 nm) profile of the ethanol extract from B. forficata leaves. Peaks 1 and 4: quercetin derivatives. Peaks 2,3,5-9: kaempferol derivatives. Peak 2: kaempferitrin.
View Figure 1
![]()
Table 1: Influence of the B. forficata extract in the hepatic metabolism of fed normoglycemic and diabetic rats.
View Table 1
Effects of BFEE on liver glycogen catabolism and glycolysis - Fed rats
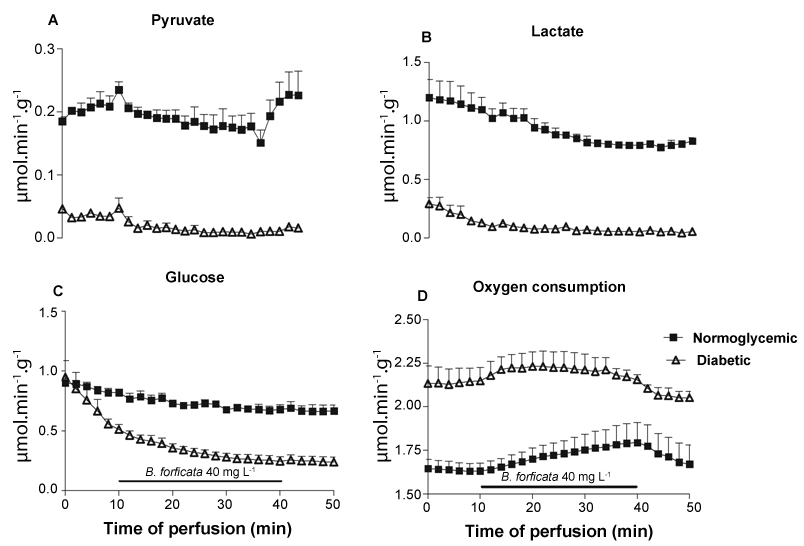
The first ex vivo experiments tested the effects of BFEE on liver glycogen catabolism and glycolysis. Liver from fed rats perfused with substrate-free medium survived at the expense of glycogen degradation via glycogenolysis and oxidation of endogenous fatty acids [23]. Under these conditions, the livers release glucose, lactate, and pyruvate because of glycogen catabolism. Figure 2 illustrates the responses of perfused livers to BFEE infusion and the experimental protocol: after a pre-perfusion period of 10 min, BFEE (40 mg L-1) was infused for 30 min, followed by an additional 10 min of extract-free medium perfusion. Four parameters were measured: glucose release, lactate and pyruvate production, and oxygen consumption. The livers of diabetic rats exhibited lower pyruvate (Figure 2A) and lactate (Figure 2B) production and increased oxygen consumption (Figure 2D) in comparison to normoglycemic rat livers. Pyruvate production was not changed by BFEE, but increased in controls when the extract infusion was ceased. Lactate production was only minimally reduced by BFEE infusion in control animals, but it was enough to cause a reduction in the lactate/pyruvate ratio (Table 1), a parameter that indicates the cytosolic NADH/NAD+ ratio. Glucose release (Figure 2C) was slightly reduced by BFEE in normoglycemic rats but exhibited a greater reduction in the diabetic rats in the presence of extract. As noted in table 1, BFEE significantly reduced glucose release by about 164% in diabetic rats and the lactate/pyruvate ratio by about 60% in diabetic rats.
.
Figure 2: Hepatic production of metabolites (μmol.min-1.g-1) in fed diabetic and normoglycemic rats (n = 3) during 50 minutes of monovascular liver perfusion. The BF extract was added to the perfusion liquid from the 10th to the 40th minute. (A) Production of pyruvate; (B) lactate; (C) glucose; and (D) oxygen consumption.
View Figure 2
![]()
Table 2: Influence of the B. forficata extract in the L-glutamine metabolism in the liver of fasting normoglycemic and diabetic rats.
View Table 2
Effects of BFEE on liver gluconeogenesis -Fasted rats
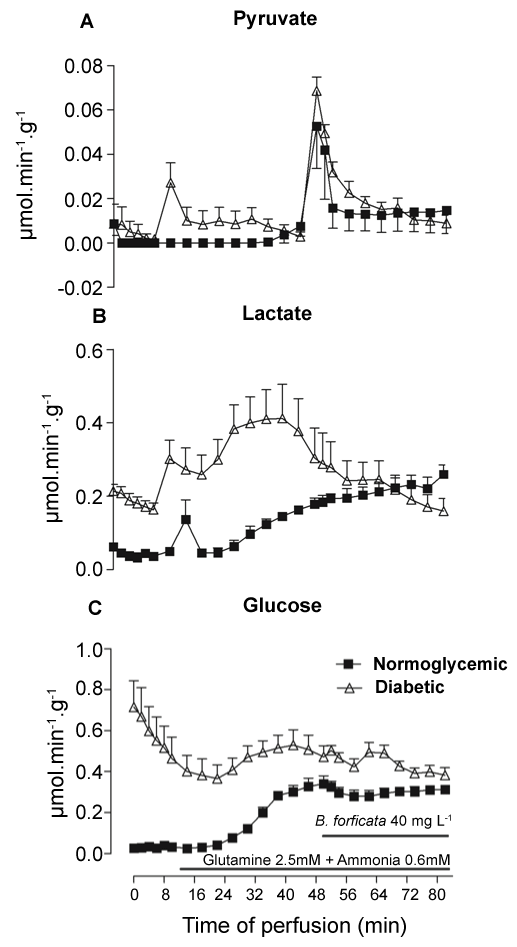
The experiments shown in figure 3 tested the ex vivo effect of BFEE on liver gluconeogenesis from L-glutamine. In order to minimize interference by glycogen catabolism, livers from 16-h fasted rats were used. After a pre-perfusion period of 10 min in the absence of substrate, 2.5 mM L-glutamine plus 0.6 mM ammonium chloride were infused for 82 min, and BFEE was added to the infusion with substrates from the 50th min. Ammonium chloride was added to accelerate L-glutamine catabolism because it stimulates glutaminase activity [24]. Diabetic animals, even in the fasted state, showed a higher basal production of glucose (Figure 3C) and lactate (Figure 3B) when compared to the normoglycemic animals. L-glutamine infusion caused an increase in glucose and lactate production in both normoglycemic and diabetic animals, but did not change pyruvate (Figure 3A) production. With L-glutamine as substrate the glucose production was only minimally increased in diabetic animals in comparison to the basal values. In contrast, the increase in lactate production was more substantial in the diabetic animals. These scenarios changed after BFEE infusion (50th min). Pyruvate production experienced a higher and transitory increment at the start of BFEE infusion in normoglycemic and diabetic animals, but rapidly returned to basal values for both conditions. Lactate production was not changed by BFEE in the controls, but the extract reduced the increment of lactate production induced by L-glutamine in diabetic animals. Both the increment and reduction in metabolites due to BFEE infusion are shown in Table 2. BFEE significantly reduced lactate production by about 60% in diabetic animals while the lactate/pyruvate ratio was reduced by 76% and 131%, respectively, for normoglycemic and diabetic animals. However, the infusion of BFEE did not significantly change glucose production.
.
Figure 3: Hepatic production of metabolites (μmol.min-1.g-1) in fasting diabetic (n = 4) and normoglycemic (n = 3) livers stimulated with the combination of L-glutamine 2.5 mM and ammonium chloride 0.6 mM. The infusion of BF extract occurred concomitantly from the 10th to the 82nd minute. (A) Production of pyruvate; (B) lactate; and (C) glucose.
View Figure 3
![]()
Table 3: Biochemical parameters (mean ± SEM) analyzed in normoglycemic or diabetic rats (n = 6) treated for 7 days with ethanolic fraction of B. forficata (300 mg kg-1) or vehicle.
View Table 3
Evaluation of the hypoglycemic potential of the BFEE
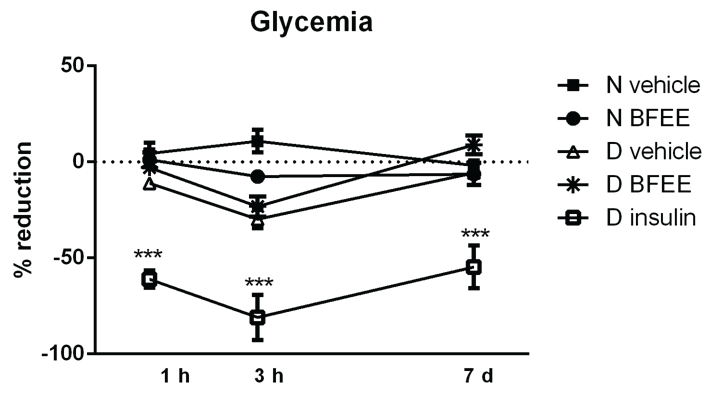
Considering the ex vivo changes observed in the hepatic metabolic pathways of glucose, we also determined the glycemia in vivo after the BFEE administration (oral) in normoglycemic and diabetic rats. The mean blood glucose of diabetic animals three days after STZ injection was 496 ± 30.3 mg dL-1. The assessment of BFEE hypoglycemic potential was conducted acutely and sub acutely in the same animals. There was no significant reduction in the blood glucose of diabetic animals after 1 and 3 h, or after 7 days of administration of 300 mg kg-1 BFEE, when compared with normoglycemic rats. However, as expected, a significant reduction in the glycemia was observed in insulin-treated rats (positive control) by ~25%, ~90%, and ~55% at 1 h, 3 h, and 7 days after treatment, respectively (Figure 4).
.
Figure 4: Changes in blood glucose of rats 1 h, 3 h and 7 days after the treatment with 300 mg Kg-1 of ethanolic extract of B. forficata, vehicle or insulin. The data are expressed as the percentage considering the pre-treatment blood glucose as 100% for each group. Comparisons were performed by two-way ANOVA. Legends: *p < 0.05, ** p < 0.01, *** p < 0.001, N = normoglycemic, D = diabetic.
View Figure 4
Biochemical parameters
Both plasmatic transaminases showed abnormalities in BFEE-treated diabetic animals; alanine- (ALT) and aspartate aminotransferase (AST) levels increased 76% and 95%, respectively, in comparison to vehicle-treated normoglycemic animals. Interestingly, the vehicle-treated diabetic rats exhibited no alteration in these enzymes. Plasma fructosamine did not differ between all groups (Table 3).
As glucose release was reduced in the perfused liver of fed diabetic rats, an in vivo experiment was performed to assess liver glycogen in these and normoglycemic animals. The results are shown in the Table 3. The liver of diabetic rats presented very low glycogen content: less than 20% of the content in the normoglycemic rats. The treatment of animals with BFEE increased liver glycogen content by about 100% in the diabetic condition, but was still less than the controls.
Histology of the pancreas and liver
Histology of the pancreas was performed to evaluate changes induced by experimental diabetes. This analysis revealed that the STZ model reduced by nearly half the number of Langerhans' islets in comparison to non-diabetic animals (Figure 5). Counting of pancreatic islets was carried out in 4 fields at 100 × magnification under a light microscope. The parameters evaluated and the scores assigned to each group are described in Table 4. There was no pancreatic difference between diabetic animals treated with extract and diabetic animals treated with vehicle.
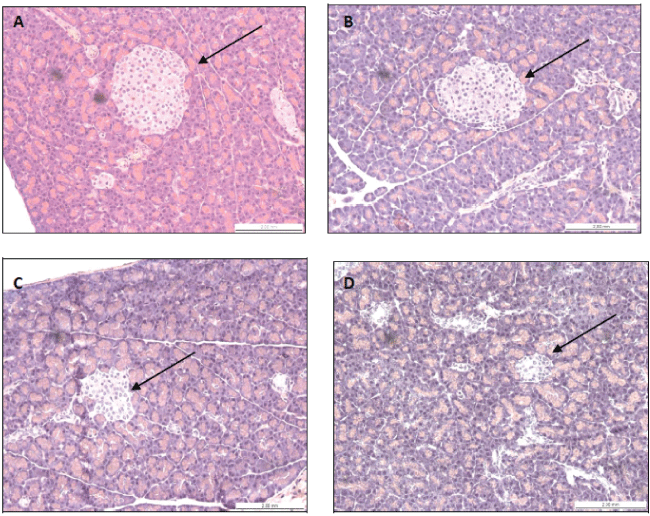
.
Figure 5: Pancreatic histology of rats treated for 7 days with the ethanolic extract of B. forficata or vehicle. The arrows indicate the islets of Langerhans in. (A) normoglycemic rats treated with vehicle; (B) normoglycemic rats treated with extract; (C) diabetic rats treated with vehicle; and (D) diabetic rats treated with the extract.
View Figure 5
![]()
Table 4: Histological parameters evaluated in the pancreas of normoglycemic or diabetic rats treated for 7 days with vehicle or B. forficata extract (300 mg kg-1).
View Table 4
Liver histology revealed no significant changes between experimental groups, indicating that neither diabetes nor the BFEE were able to produce alterations in the microscopic features of this organ (data not shown).
Changes in body weight
To assess whether BFEE influences body weight, animals were weighted at the beginning and after 7 days of treatment. The body weight gain of the normoglycemic group (17.3 ± 2.1 g) was higher than in diabetic rats (12.9 ± 2.4 g), and the administration of BF extract did not improve this parameter in diabetic animals (8.2 ± 1.1 g).
Discussion
The plants of the genus Bauhinia sp., especially B. forficata, are popularly identified as natural hypoglycemic agents, indicated for the treatment of diabetes; however, scientific data regarding this and other activities are scarce in the literature. Some authors have investigated the hypoglycemic effect of B. forficata [8,9,12,25], but the glucose pathways into the liver were not studied. This is the first report of the effects of B. forficata extract in the hepatic metabolism of glucose. We evaluated the effects of B. forficata ethanolic extract (BFEE) on glycogenolysis and glycolysis in perfused liver of fed rats; while perfused liver of fasted rats were stimulated with L-glutamine to investigate the effects of BFEE on gluconeogenesis.
Significant metabolic differences were observed between diabetic and normoglycemic rats in fasted and fed states. The liver of perfused diabetic rats exhibited higher glucose production under fasting conditions (Figure 3C), but similar production of glucose under fed conditions (Figure 2C) when compared to normoglycemic rats in the beginning of liver perfusion. Glycolysis was also reduced in the diabetic condition, as evidenced by the decrease in lactate (Figure 2B) and pyruvate (Figure 2A) release. Then increased oxygen consumption in diabetic rats could be the result of compensatory energy production from the aerobic consumption of fatty acids. Glucose levels in fed rats are compatible with the reduced glycogen level found in the liver of diabetic rats (Table 3). In the absence of insulin, glycogen synthesis ceases and glucogenolysis begins; thus, more glucose is exported from the liver to the blood, increasing hyperglycemia even more [26]. Low glycogen accumulation has also been described in STZ-induced diabetic rats [25,27-29]. Liver perfusion reduced glucose production more quickly in diabetic rats than in normoglycemic rats, probably because the control livers released glucose from accumulated glycogen (glycogenolysis), while diabetic livers release glucose via gluconeogenesis through endogenous substrate.
The infusion of BFEE in the perfused liver of fed diabetic rats reduced glucose release by 63%, but it did not affect glycolysis, represented by lactate and pyruvate released (Table 1). Thus, the reduction in the glucose release seems to be a consequence of the depletion of glycogen stores, which were very low in these animals. The lower production of hepatic pyruvate and lactate in diabetic animals was not modified by BFEE infusion.
The effects of BFEE infusion on glucose production in fasted rat livers were less extensive than in fed condition. Since the basal glucose release (Figure 3C) in fasted diabetic rats (0.71 ± 0.12 μmol min g-1) was significantly higher than in normoglycemic rats (0.027 ± 0.01 μmol min g-1) and the glycogen content was very low (Table 3), the released glucose is probably the product of increased gluconeogenesis. Thus, the perfused liver releases a considerable concentration of amino acids from proteolysis, which is enough to sustain gluconeogenesis in the diabetic rat liver [30]. For this reason, the increment in glucose production triggered by L-glutamine in diabetic rats was significantly lower than in normoglycemic rats, since its basal level was already elevated. However, concomitant infusion of BFEE with L-glutamine did not change glucose production in normoglycemic and diabetic fasted rats. Thus, the suggestion of Pepato et al. [9] that B. forficata could act in gluconeogenesis as the biguanines do was not confirmed by our data, at least when L-glutamine was the gluconeogenic source.
The livers of rats with type 1 diabetes respond differently to glutamine, and the differences are characterized mainly by an acceleration of metabolic flux during the initial stages of the amino acid infusion [31]. Accordingly, the increment in lactate production triggered by L-glutamine was higher in diabetic rats (Figure 3B). BFEE infusion inhibited lactate production and stimulated pyruvate production; thus, the extract altered the cytosolic redox state since this indicated an accentuated reduction in the NADH/NAD+ ratio. Our data could suggest that the higher lactatemia in diabetes is, at least in part, originating from the hepatic metabolism of amino acids such as L-glutamine.
In despite of the reduction in glucose production induced by BFEE infusion in the livers of fed diabetic rats (Table 1), glycemia did not change, either acutely or subacutely (Figure 4). The scientific evidence for the B. forficata effects on glycemia is controversial. Our results corroborate those of Coimbra-Teixeira et al. [15] who used B. forficata alcoholic extract and those of Volpato et al. [16] who used an aqueous extract; however, our results differ from those reported by Menezes et al. [32], Silva et al. [11] and Curcio et al. [12]. The last authors observed hypoglycemia after treatment with 500 and 600 mg kg-1 of B. forficata n-butanol fraction, and 800 mg kg-1 of BF leaf extract, respectively. These doses, however, are higher than that used in our work (300 mg kg-1). Reinforcing our results, the dose of 400 mg kg-1 also failed to reduce the glycemia in diabetic rats [11].
The anti-diabetic activity of B. forficata has been associated with the content of flavonoids glycosides, mainly kaempferitrin [8,33]. In order to get an extract rich in glycosyl flavonoids, the leaves of B. forficata were extracted with hexane, dichloromethane and ethyl acetate before extraction with ethanol. Therefore, ethanol extract (BFEE) was obtained free of less polar constituents. In agreement, the analyses by HPLC-DAD showed the presence mainly of kaempferol glycosides, including kaempferitrin (Figure 1, peak 2), considered the chemical marker of the species. However, this active compound is not a major flavonoid in the studied sample, as can be seen in the figure 1. Considering the high glycemia observed in the diabetic rats (496 ± 30.3 mg dL-1), it is probable that the content of kaempferitrin in the BFEE was insufficient to reduce such high glycemic levels. This hypothesis is corroborated by observation that kaempferitrin is 106-fold less potent than insulin [13]. Furthermore, evaluation of α-glucosidase inhibitory activity of B. forficata extracts led to conclusion that kaempferol derivatives are less active than quercetin derivatives [7]. As showed above, BFEE is richer in kaempferol derivatives, what can explain our results.
Seven days BFEE treatment of diabetic rats was not able to assist these animals to gain weight as the control group. Body weight must be evaluated in patients with uncontrolled diabetes, as well as the plasmatic transaminases; these parameters are markers of the progression of uncontrolled diabetes to metabolic syndrome [34,35]. Our data showed that the BFEE and STZ-induced diabetes in isolation did not alter transaminase activity, while the combination of these factors significantly increased ALT and AST. In contrast, plasmatic fructosamine did not change with BFEE treatment. This result was expected since fructosamine forms glycated serum proteins [36], thus inferring changes in glycemia over a period of two to three weeks, a longer time than our experiment (one week).
Conclusions
Considering the whole data we conclude that BFEE interferes with hepatic glycolysis, glucogenesis, and lactate production from L-glutamine. Thus the BFEE effects depend of the alimentary state. BFEE treatment caused a minimal increase in the liver glycogen content of diabetic rats, not enough to restore the animals to control levels, and with no change in glycemia. Since this is the first study to demonstrate the effects of Bauhinia forficata on hepatic metabolism, additional, longer-term studies are necessary to better assess its metabolic effects, including analysis of sources of gluconeogenesis other than L-glutamine. Also is necessary more chemical investigations of B. forficata concerning factors that affect the secondary metabolite production as season, environmental conditions, and genetic.
Competing Interests
The authors declare no conflict of interest.
Acknowledgements
This research was supported by Fundação Araucaria (438/2012 and 21/2012). The authors are grateful to CAPES, CNPq and Fundação Araucária for the fellowships.
Ethical Approval
All protocols were approved by the Ethics Committee for Animal Experimentation of the Biological Science Sector of UFPR (certificate number 577) and performed according to the international guidelines regarding the ethical use of laboratory animals.
References
-
Kerner W, Brückel J, German Diabetes Association (2014) Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 122: 384-386.
-
Janapti YK (2015) Optimize diabetes by herbal medicine: a review. Journal of Advances in Medical and Pharmaceutical Sciences 3: 2394-1111.
-
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94: 311-321.
-
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4-14.
-
Silva KL, Biavatti MW, Leite SN, Yunes RA, Delle Monache F, et al. (2000) Phytochemical and pharmacognositc investigation of Bauhinia forficata Link (Leguminosae). Z Naturforsch C 55: 478-480.
-
Pizzolatti MG, Anildo Cunha Jr , Szpoganicz B, Sousa E, Braz-Filho R, et al. (2003) Flavonoids glycosides from leaves and flowers of Bauhinia forficata (Leguminosae). Quim Nova 26: 466-469.
-
Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentão P, et al. (2012) Bauhinia forficata Link authenticity using flavonoids profile: relation with their biological properties. Food Chem 134: 894-904.
-
Marques GS, Rolim LA, Alves LDS, Silva CCAR, Soares LAL, et al. (2013) Estado da arte de Bauhinia forficata Link (Fabaceae) como alternativa terapêutica para o tratamento de diabetes mellitus. Rev Ciênc Farm Básica Apl 34: 313-320.
-
Pepato MT, Keller EH, Baviera AM, Kettelhut IC, Vendramini RC, et al. (2002) Anti-diabetic activity of Bauhinia forficata decoction in streptozotocindiabetic rats. J Ethnopharmacol 81: 191-197.
-
Lino Cde S, Diógenes JP, Pereira BA, Faria RA, Andrade Neto M, et al. (2004) Antidiabetic activity of Bauhinia forficata extracts in alloxan-diabetic rats. Biol Pharm Bull 27: 125-127.
-
Silva FR, Szpoganicz B, Pizzolatti MG, Willrich MAV, de Sousa E (2002) Acute effect of Bauhinia forficata on serum glucose levels in normal and alloxan-induced diabetic rats. J Ethnopharmacol 83: 33-37.
-
Curcio SA, Stefan LF, Randi BA, Dias MA, da Silva RE, et al. (2012) Hypoglycemic effects of an aqueous extract of Bauhinia forficata on the salivary glands of diabetic mice. Pak J Pharm Sci 25: 493-499.
-
Jorge AP, Horst H, de Sousa E, Pizzolatti MG, Silva FR (2004) Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem Biol Interact 149: 89-96.
-
Tzeng YM, Chen K, Rao YK, Lee MJ (2009) Kaempferitrin activates the insulin signaling pathway and stimulates secretion of adiponectin in 3T3-L1 adipocytes. Eur J Pharmacol 607: 27-34.
-
Coimbra-Teixeira C, Danni FD, Blotta RM, Mussnich DG, Pereira da Costa P, et al. (1992) Plants employed in the treatment of diabetes mellitus: results of an ethnopharmacological survey in Porto Alegre, Brazil. Fitoterapia 63: 320-322.
-
Volpato GT, Damasceno DC, Rudge MV, Padovani CR, Calderon IM (2008) Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J Ethnopharmacol 116: 131-137.
-
Levinthal GN, Tavill AS (1999) Liver disease and diabetes mellitus. Clin Diabetes 17: 1-20.
-
Schreiber AK, Neufeld M, Jesus CH, Cunha JM (2012) Peripheral antinociceptive effect of anandamide and drugs that affect the endocannabinoid system on the formalin test in normal and streptozotocin-diabetic rats. Neuropharmacology 63: 1286-1297.
-
Bracht A, Ishii-Iwamoto EL, Kelmer-Bracht AM (2003) O estudo do metabolismo no fígado em perfusão. In: Bracht A, Ishii-Iwamoto EL. Métodos de laboratório em Bioquímica. São Paulo: Manole 275-288.
-
Bergmeyer HU (1974) Methods of enzymatic analysis, Verlag Chemie-Academic Press, New York.
-
Kepler D, Decker K.Glycogen (1974) determination with amyloglucosidase. In: Bergmeyer HU. Methods of enzymatic analyses. New York: Verlag Chemie-Academic Press 1126-1131.
-
Culling CFA, Allison RT, Barr WT (1985) Cellular Pathology Technique. London: Butterworth and Co.
-
Scholz R, Bücher T (1965) Hemoglobin-free perfusion of rat liver. In: Chance B, Estabrook RW, Williamson JR. Control of Energy Metabolism. New York: Academic Press 393-414.
-
Comar JF, Suzuki-Kemmelmeier F, Constantin J, Bracht A (2010) Hepatic zonation of carbon and nitrogen fluxes derived from glutamine and ammonia transformations. J Biomed Sci 17: 1-11.
-
da Cunha AM, Menon S, Menon R, Couto AG, Bürger C, et al. (2010) Hypoglycemic activity of dried extracts of Bauhinia forficata Link. Phytomedicine 17: 37-41.
-
Salway JG (2009) Metabolismo passo a passo. 3 ed. (Metabolism at a Glance. 3rd ed.) Porto Alegre: Artmed 110-119.
-
Haritha C, Reddy AG, Reddy YR, Anjaneyulu Y, Rao TM, et al. (2013) Evaluation of protective action of fenugreek, insulin and glimepiride and their combination in diabetic Sprague Dawley rats. J Nat Sci Biol Med 4: 207-212.
-
Marzouk M, Soliman AM, Omar TY (2013) Hypoglycemic and antioxidative effects of fenugreek and termis seeds powder in streptozotocin-diabetic rats. Eur Rev Med Pharmacol Sci 17: 559-565.
-
Bavarva JH, Narasimhacharya AV (2013) Systematic study to evaluate anti-diabetic potential of Amaranthus spinosus on type-1 and type-2 diabetes. Cell Mol Biol (Noisy-le-grand) 59 Suppl: OL1818-1825.
-
Zwiebel FM (1990) Der mitochondriale monocarboxylat-translocator. PhD Thesis. University of Munich, Munich 308.
-
de Oliveira DS (2006) Some features of the livers of rats with type 1 diabetes and its metabolic responses to glutamine.Master Dissertation. Graduate Program in Biological Sciences, UEM, Maringá, Brazil.
-
Menezes FS, Minto ABM, Ruela HS, Kuster RM, Sheridan H, et al. (2007) Hypoglycemic activity of two Brazilian Bauhinia species: Bauhinia forficata L. and Bauhinia monandra Kurz. Rev Bras Farmacogn 17: 08-13.
-
Sousa E, Zanatta L, Seifriz I, Creczynski-Pasa TB, Pizzolatti MG, et al. (2004) Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)- dirhamnoside from Bauhinia forficata leaves. J Nat Prod 67: 829-832.
-
Saligram S, Williams EJ, Masding MG (2012) Raised liver enzymes in newly diagnosed Type 2 diabetes are associated with weight and lipids, but not glycaemic control. Indian J Endocrinol Metab 16: 1012-1014.
-
Xourafas D, Ardestani A, Ashley SW, Tavakkoli A (2012) Impact of weight-loss surgery and diabetes status on serum ALT levels. Obes Surg 22: 1540-1547.
-
Armbruster DA (1987) Fructosamine: structure, analysis, and clinical usefulness. Clinical Chemistry 33: 2153-2163.





