International Journal of Diabetes and Clinical Research
Evaluation of a Standardized Inpatient Insulin Therapy Based on Electronic Insulin Dose Calculation - A before after Cohort Proof of Concept Study
Rahel Nussbaumer1, Philipp Schuetz1, Beat Mueller1, Robert Thomann2 and Anne Katrin Borm1*
1Kantonsspital Aarau, Aarau, Switzerland
2Buergerspital Solothurn, Solothurn, Switzerland
*Corresponding author:
Anne Katrin Borm, University Hospital of Medicine, Division of Endocrinology, Diabetes and Metabolism, Kantonsspital Aarau,Tellstrasse, CH-5001 Aarau, Switzerland, Tel: + 41 62 838 68 12, Fax: + 41 62 838 98 73, E-mail: annekatrin.borm@ksa.ch
Int J Diabetes Clin Res, IJDCR-3-060, (Volume 3, Issue 2), Original Research Article; ISSN: 2377-3634
Received: February 21, 2016 | Accepted: May 27, 2016 | Published May 31, 2016
Citation: Nussbaumer R, Schuetz P, Mueller B, Thomann R, Borm AK (2016) Evaluation of a Standardized Inpatient Insulin Therapy Based on Electronic Insulin Dose Calculation - A before after Cohort Proof of Concept Study. Int J Diabetes Clin Res 3:060. 10.23937/2377-3634/1410060
Copyright: © 2016 Nussbaumer R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Diabetes is a common comorbidity in hospitalized patients. The necessity of blood glucose control in those patients with low variability and avoidance of hypoglycemic episodes is well-known. Yet, there is still only marginal literature about the optimal therapy of hyperglycemia in non critically ill hospitalized patients using tools of modern electronic patients charts.
Objectives: The present study aimed to investigate the feasibility, the safety and efficiency of a standardized inpatient insulin therapy based on a new electronic insulin dose calculating program.
Patients and methods: In this retrospective study with a before-after design, we compared patients treated with the new electronic insulin protocol (study group) to a historical group treated with a traditional paper-based sliding scale insulin protocol (SSI). Patients were selected with the aid of the hospital's electronic medical system. Inclusion criteria for patients were either a lower respiratory tract infection or an acute cardiac condition with diabetes as a known comorbidity. Blood glucose levels were measured four times a day during the first 120 in-hospital hours. In the study group, all included patients were treated with the new electronic insulin protocol, which calculated an appropriate dose of short-acting insulin considering actual blood glucose, ingested carbohydrates and a defined resistance factor.
Results: Patients treated with the electronic insulin protocol were compared to a matched historical control group referring to the mean glucose levels, hypoglycemia and variability of blood glucose levels. While mean glucose levels were equal between the two groups (8.65 to 8.70), there was a trend towards less hypoglycemic episodes (1.26% to 1.46%, p = 0.120) and a significant smaller variability (6.36 to 8.75, p = 0.025) in patients treated with the electronic insulin protocol.
Conclusion: In a real-life setting an electronic insulin protocol may improve and simplify glycemic control with less variability and a trend towards less hypoglycemic events among non-critically ill hospitalized patients.
Keywords
Electronic insulin protocol, Glycemic control, Hypoglycemia, Standardized insulin therapy
Background
Mal treated hyperglycemia is associated with increased mortality and morbidity across different patient population and clinical settings. Although most literature refers to patients in critical care settings, similar effects may be found in non-critical care medical wards [1-16].
Not only hyperglycemia is responsible for adverse clinical outcomes, hypoglycemia also increases the risk of mortality [8,9,17-23]. Additionally, high glycemic variability has also been recognized as a poor prognostic factor in regard to hospital outcomes [24-31]. Recent studies suggest that in fact all three aspects of glycemic control-hyperglycemia, hypoglycemia and glycemic variability need to be taken into account for optimizing glycemic control [32,33]. Still, there are only few studies investigating ways to improve glycemic control in medical wards in a real-life setting. In addition, electronic patient charts are increasingly present in hospitals daily routine. This offers an opportunity for supportive user-oriented tools that calculate insulin dosage based on blood glucose level, ingested carbohydrates and the assumed level of insulin resistance.
Objectives
In April 2013, we introduced a new standardized electronic system for glycemic control. For this purpose, we recently developed an electronic bolus calculator, using correction factors for actual blood glucose, carbohydrate (CH) intake and a resistance factor.
The purpose of the current study was to assess the feasibility, the safety and quality of this new electronic system for the 3 aspects of glycemic control (hyperglycemia, hypoglycemia and glycemic variability) as compared to a historical control group from our hospital.
Methods
Patients and ethics
In this study with a before-after design we compared comparable medical ward in patients who were treated with the new electronic insulin protocol from 1 July 2013-31 July 2014 to those hospitalized from 1 January - 31 December 2008 managed with a traditional glucose-adapted insulin sliding scale (SSI), the former standard of care (historical controls). The methodology of the new electronic insulin protocol is similar to a former study and was reported elsewhere [34].
The study was approved by the local ethical board (Kantonale Ethikkommission Aargau). The study and lack of patient interventions, the study was classified as a quality-control project and need for written informed consent had been waived by the ethical board.
Patients were selected based on hospital's electronic medical record system search. Eligible patients were those with diabetes mellitus type 1 or type 2 as a comorbidity and with the main diagnosis of either 1) a lower respiratory tract infection (LRTI) including pneumonia or chronic obstructive pulmonary disease exacerbation, or 2) an acute cardiac condition including acute coronary heart disease (ST-elevation or non-ST-elevation myocardial infarction, unstable angina pectoris) or acute heart failure. Both, causes of admission and diabetes as a comorbidity were identified based on medical records.
Patients with a new diagnoses of diabetes at or after the index admission and patients with diabetes as the primary cause of admission were not included. In addition, we excluded patients if (a) the electronic insulin protocol was not started within the first 48 hours of admission, (b) insufficient number of blood glucose measurement during observation time (at least 2 measurements per day in the first 120 inpatients hours), or (c) hospitalization in the medical ward was < 5 days, due to early discharge from the hospital or transfer to the ICU during the first 120 inpatient hours, because the electronic insulin protocol was not used in either the outpatient or critical care settings.
Glucose measurement and insulin treatment
In all patients, glucose levels were recorded and insulin therapy was initiated as directed by the treating physician team according to the hospital insulin protocol. Glucose levels were measured 4 times a day, three times before meals and once before bed-time.
During the hospital stay, the insulin treatment in both groups consisted of once or twice daily doses of long-acting insulin, generally Levemir® (insulin detemir, Novo Nordisk, Bagsvaerd, Denmark) or, Lantus® (insulin glargine, Sanofi, Paris, France), plus pre-prandial corrective doses of short-acting insulin, either Humalog® (insulin lispro, Lilly, Indianapolis, IN, USA) or NovoRapid® (insulin as part, Novo Nordisk).In the historical control group, the dose of short acting insulin and basal insulin was up to the provider's decision on a day to day
Electronic insulin protocol
The electronic insulin protocol was introduced in 2013, after the electronical patient chart was established at the Medical University Clinic at the Kantonsspital Aarau. The electronic insulin protocol is based on a bolus calculator for short-acting insulin.
On admission of a diabetic patient, physicians prescribed the amount of carbohydrate content in the nutrition, a "resistance factor" (described in the following section) and the dose of basal insulin. The nurse was asked to enter the actual blood glucose and the amount of carbohydrates the patient would probably ingest into the software.
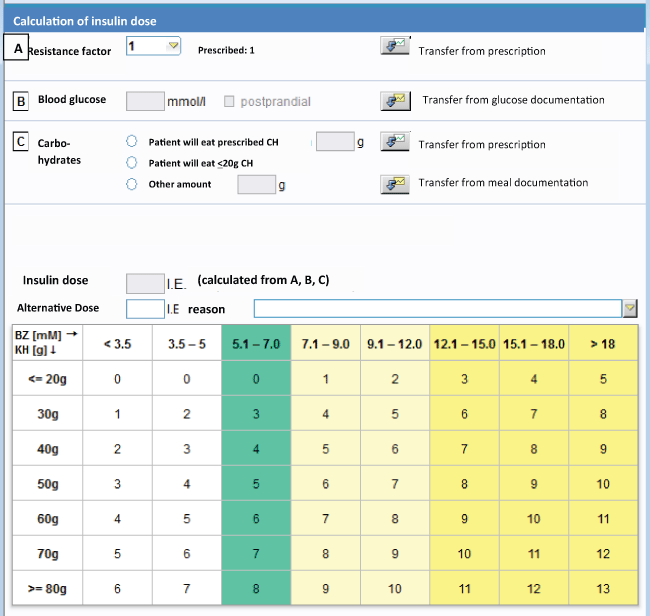
.
Figure 1: Insulin protocol incorporating insulin resistance, blood glucose and carbohydrate content of the nutrition for the calculation of insulin dose. Calculation of total rapid insulin dose: Total insulin dose = resistance factor A * (B + C)
View Figure 1
The software calculates the dose of bolus insulin by three factors for dosing insulin (Figure 1):
A = correction factor (correction of the latest blood glucose level)
A = (actual blood glucose - 7)/2
1E of short acting insulin is used for a correction of 2 mmol/l with a target of 7 mmol/l
B = carbohydrate factor (factor for the correction of the amount of ingested carbohydrates)
B = Amount of ingested carbohydrates/10
1E short acting insulin is applied to cover 10 g carbohydrates
C = resistance factor (RF)
The resistant factor is a multiplicator and stands for the degree of insulin resistance.
The resistance factor has been implicated to simplify this insulin protocol, so that there was no need to prescribe both correction factor and carbohydrate factor. Physicians are asked to start with an RF = 1 in normal weight patients. In patients with criteria, which lead to the assumption of higher insulin resistance, e.g. obesity (BMI > 30), sepsis or elevated inflammatory analytes (CRP > 100 mg/dl), or in patients on higher pre-admission insulin doses (> 60 E Insulin/d), it is recommended, to start directly with RF 3. The RF as a marker of insulin resistance is adjusted daily in stepwise fashion-it is rised if insulin doses failed to correct hyperglycemic glucose levels, and has to be reduced if insulin doses are associated with hypoglycemic glucose concentrations or a dramatic decrease in glucose levels ( ≥ 50%).
The suggested dose of short acting insulin is calculated using this formula:
Insulin dose = C* (A+B).
Before implementation of this electronic insulin protocol, the staff of the hospital kitchen was trained to weight the food of the patients to assure correct distribution of prescribed carbohydrates. A corresponding, standardized paper-based insulin protocol using the same factors had been introduced in 2009 and had been used from 2009- 2013 in our hospital. Staff taking care of diabetic patients are trained on this insulin protocol regularly.
SSI (Historical control protocol)
The SSI algorithm called for a set amount of insulin to be administered based only on the patient's latest blood glucose level, without considering the timing or carbohydrate content of meals or snacks, the pre-admission insulin regimen, or the degree of insulin resistance. Patients received their usual home doses of basal insulin. If they were not on basal insulin, it was started during the hospitalization based on the decision of their physician.
With both, the electronic insulin protocol and the SSI, the treating physician team were permitted to modify or disregard the protocol based on clinical judgment.
Endpoints
The primary endpoints were 1) the mean glucose level, 2) variability of blood glucose levels and 3) frequency of occurrence of hypoglycemic events. All three endpoints were examined over the first 120 inpatient hours. The mean glucose level over the first 120 hours was determined as the average of all available glucose concentration measurements within that period, assuming a linear trend between those measurements.
Secondary endpoints included hospital length-of-stay, the incidence of all-cause mortality after the first 120 inpatient hours and transfer to the ICU during hospital stay.
Statistical Analysis
To describe the populations, values are expressed as means with standard deviations (SDs) and frequencies as percentages, counts or both, as appropriate. We investigated differences in endpoints between the electronic group and the historical control group with linear and logistic regression analysis adjusted for important confounders, namely age, diabetes type, medical therapy on admission (metformin, insulin), and main hospital diagnosis (LRTI or acute cardiac condition). We estimated glucose variability across all measured blood glucose levels per patient using Excel's variance function.
In a second step, we investigated factors potentially associated with hypoglycemia in the overall study population using logistic regression analysis and stepwise selection procedures at the p < 0.2 limit. All analyses were performed with Stata 12 (StataCorp, College Station, TX, USA).
Results
Study participants
A majority of the 223 patients included had type 2 diabetes (216/223, 96.9%). Out of these 223 patients, 136 (61%) were in the group with the electronic insulin protocol and 87 (39%) in the historical control group. The main cause for hospitalization was an acute cardiac condition in 151 patients (67.7%) followed by LRTI in 72 (32.3%).
Table 1 shows the baseline characteristics separated into the two cohorts. The two cohorts are well matched with regard to most socio-demographic and metabolic variables related to diabetes. The interventional group consisted of older patients with a higher body mass index. Also, while in the historical control group the principal cause for hospitalization was equally distributed with LRTI in 44 (50.2%) an acute cardiac condition in 43 (49.8%) cases, the patients in the group with the electronic insulin protocol were predominantly hospitalized because of acute cardiac conditions (108/136, 79.4%).
![]()
Table 1: Baseline characteristics in the overall study sample and according to treatment group.
View Table 1
Differences in glycemic control
In comparison to the historical group, patients in the group with the electronic insulin protocol had similar mean glucose values (8.7 ± 2.23 vs. 8.65 ± 1.58, -0.08 (95% CI -0.61 to 0.46)). The frequency of hypoglycemic episodes was similar in the two groups (1.46% vs. 1.26%, 0.42 (95% CI 0.14 to 1.25)). Yet, the glycemic variability was lower in the electronic insulin protocol group (8.75 mmol/l vs. 6.38 mmol/l) This was confirmed after adjustment for age, diabetes type, main hospital diagnosis (LRTI or acute cardiac condition) and chronic therapy at admission (metformin, insulin) with a linear regression coefficient of -2.63 (95% CI -4.93 to -0.33). Detailed results of all outcomes are presented in (Table 2). Table 3 shows the distribution of the blood glucose values measurements during the first 120 inpatient hours. While the measured values of the control group are nearly equally distributed, clearly more values of the study group are in the target range of 7.1-10.0 mmol/L.
![]()
Table 2: Primary and secondary outcomes: adjusted regression coefficient or odds ratio for electronic insulin protocol group to historical control group to historical control group.
View Table 2
![]()
Table 3: Distribution of patients across blood glucose ranges in the two groups during the first 120 inpatient hours.
View Table 3
In hospital mortality and transfer to ICU have been lower in the study group compared to the historical control group (p < 0.05).
Discussion
Using a before-after design and focusing on two specific patient populations, this proof-of-concept study found a reduction in glucose variability and a trend towards lower risk for hypoglycemia with similar mean glucose levels when an electronic insulin protocol was used for insulin titration in hospitalized medical inpatients as compared to a more traditional SSI algorithm.
It is well established that well controlled blood glucose in hospitalized patients is an important protective factor for mortality and morbidity with substantial impact on wound healing and motivation for diabetes therapy [8,9,35]. In addition, recent studies support the association between recurrent episodes of hypoglycemia and adverse clinical outcomes, such as increased mortality, multi organ failure, systemic infections and a prolonged length of hospitalization [20,36]. International guidelines recommends premeal target of less than 7.8 mmol/L and a random blood glucose level of less than 10 mmol/L for the majority of hospitalized patients with non-critical illness [16]. ADA-Guidelines suggest a blood glucose level between 7.8-10 mmol/L [37]. Importantly, these targets should be reached without increasing the risk for hypoglycemia and high glucose variability. To achieve this goal, smart insulin titration schemes are urgently needed for routine clinical care.
The management of hyperglycemia should be individually adjusted for each patient, considering other comorbidities and the risk factors for hyperglycemia [38]. Acute disease related stress and medication (steroids) in hospitalized patients induces insulin resistance is, if left untreated, associated with hyperglycemia.
At the same time an overly simplified dosage of insulin using traditional sliding scales, neglecting variable carbohydrate intake of meals and insulin-resistance control may result in hypoglycemia, which is identified as an independent risk factor for mortality [37].
Additionally, increased glycemic variability should be reduced due to its association with higher mortality which may be explained by its effect on increasing oxidative stress [39].
Yet, in clinical routine, achieving these goals is challenging. Patient's nutritional status, level of consciousness and teaching as well as the practical limitations of glycemic monitoring have an effect on blood glucose levels [37]. A fine balance in glycemic management is important to reduce risks for adverse clinical outcomes but at the same keep glucose levels in the target range [38]. Only few research studies focus on the question about optimal handling of blood glucose or how to reach the best possible blood glucose control in hospitalized patients. The widespread use of sliding scale insulin (SSI) has never been well studied in the context of improved clinical outcomes. In fact, several studies found no benefit from the use of SSI, since SSI are only reactive strategies and do not incorporate the individual insulin sensitivities or changes in insulin sensitivity due to the different periods of acute illness [40-43].
In an attempt to optimize in hospital blood glucose control, we tested a new electronic program for the calculation of the dosage of short acting insulin and blood glucose management, which was developed and introduced in the Kantonsspital Aarau, relating to safety and efficiency of glycemic control. Although the sample size of the two groups is limited, we documented an improvement of glycemic control, namely a lower glycemic variability. In our study, we found a trend to less hypoglycemic episodes leading to an increased patients safety using the standardized electronic scheme. Even though we assume that many hospitals are increasingly using comparable software's for insulin management we could not find any research study focusing on electronic insulin protocols.
We also analysed the in-hospital mortality and the transfer to the ICU, which were significantly lower in the study group. It is well known, that adequate glucose control is associated with lower risk for mortality and adverse clinical outcomes. Since the study was not randomized-controlled, causal inference is difficult to establish. Thus differences in patient outcomes may also be attributable to changes in the hospital logistics and seasonal changes in the patient population. In fact, the Medical University Hospital of the Kantonsspital Aarau AG has made big efforts to optimize patient processes for discharge and patient monitoring which has been shown to significantly reduce length of stay and improve quality of care [44-49].
We tried to compare homogenous groups. However, due to the retrospective design, many changes in the treatment regimen have been undertaken between 2008 and 2013. The patients of the control group have been hospitalized in 2008. At that time, frequency of blood glucose measurements was given on a provider´s decision on a day to day basis. Therefore, we had many dropouts in the historical control group because of insufficient data. The treatment of pneumonia has changed towards more ambulatory treatment, hence less patients with pneumonia have been hospitalized in 2013 compared to 2008. Furthermore, the use of sulfonylurea was lower in the study group, since in 2013 new oral and GLP1-agonists have been available in 2008. Oral anti diabetics and GLP1-Agonists have more often been stopped during hospitalization with functional insulin therapy in 2013. Though our efforts to have comparable groups, our study has the limitation that there were mismatches concerning the cause of hospitalization and the use of oral anti diabetics. Since we investigated the feasibility and the safety of the electronic insulin dosing scheme, this should not influence the outcome of the study.
As proof-of-concept study there are further limitations to be mentioned. First, the analysis is based on retrospectively collected data in the historical control group. This may lead to a selection bias due to the fact that, patients with reasonable blood glucose values were excluded because of lack of measurements. Second, the glucose control in the historical control group was already on a high level of quality as compared to the literature [50] and differences to the new scheme may be small. Yet, the resulting bias would be conservative. Third, to limit heterogeneity of our complex polymorbid patient cohort, we focused on two distinct populations. Thus, our data may not be generalizable in other populations. Forth, as a proof-of-concept study our data are rather hypothesis-generating and need to be validated in a multicenter setting including a more representative, heterogeneous patient cohort.
Conclusion
An electronic insulin protocol may improve blood glucose control with smaller glycemic variability and a trend toward less hypoglycemia. As the patient population gets older and sicker and hyperglycemia more frequent in the in hospital setting, it is important to have well designed electronic tools to ensure glycemic control during hospital stay. Toward this aim, the studied electronic insulin protocol is a first step to standardize, optimize and simplify the in-hospital blood glucose management in real-life.
Acknowledgement
The electronic insulin protocol has been developed together with Cistec AG, Zurich, suitable to our clinic information system KISIM©. We thank M. Heckendorn, Cistec AG for the excellent collaboration.
References
-
Foo K, Cooper J, Deaner A, Knight C, Suliman A, et al. (2003) A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart 89: 512-516.
-
Krinsley JS (2003) Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 78: 1471-1478.
-
Cooper MS, Stewart PM (2003) Corticosteroid insufficiency in acutely ill patients. N Engl J Med 348: 727-734.
-
Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenstrom A, et al. (1996) Effects of insulin treatment on cause-specific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. DIGAMI Study Group. Diabetes Insulin-Glucose in Acute Myocardial Infarction. Eur Heart J 17: 1337-1344.
-
Malmberg KA, Efendic S, Ryden LE (1994) Feasibility of insulin-glucose infusion in diabetic patients with acute myocardial infarction. A report from the multicenter trial: DIGAMI. Diabetes Care 17: 1007-1014.
-
Malmberg K, Norhammar A, Wedel H, Ryden L (1999) Glycometabolic State at Admission: Important Risk Marker of Mortality in Conventionally Treated Patients With Diabetes Mellitus and Acute Myocardial Infarction Long-Term Results From the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) Study. Circulation 99: 2626-2632.
-
Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML (2009) Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 37: 3001-3009.
-
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, et al. (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345: 1359-1367.
-
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, et al. (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354: 449-461.
-
der Voort PHJ, Feenstra RA, Bakker AJ, Heide L, Boerma EC, et al. (2006) Intravenous glucose intake independently related to intensive care unit and hospital mortality: an argument for glucose toxicity in critically ill patients. Clin Endocrinol (Oxf) 64: 141-145.
-
Malmberg K (1997) Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ 314: 1512-1515.
-
Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, et al. (1995) Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): Effects on mortality at 1 year. J Am Coll Cardiol 26: 57-65.
-
Dungan KM, Braithwaite SS, Preiser JC (2009) Stress hyperglycaemia. Lancet 373: 1798-1807.
-
Schuetz P, Castro P, Shapiro NI (2011) Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care 34: 771-778.
-
Schuetz P, Kennedy M, Lucas JM, Howell MD, Aird WC, et al. (2012) Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med 125: 670-678.
-
Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, et al. (2012) Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 97: 16-38.
-
Bagshaw SM, Egi M, George C, Bellomo R, Australia New Zealand Intensive Care Society Database Management Committee (2009) Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med 37: 463-470.
-
Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, et al. (2006) Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med 34: 2714-2718.
-
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, et al. (2010) Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 85: 217-224.
-
Finfer S, Liu B, Chittock DR, Norton R (2012) Hypoglycemia and risk of death in critically ill patients. N Engl J Med 367: 1108-1118.
-
Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, et al. (2010) Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 38: 1430-1434.
-
Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, van Braam Houckgeest F, et al. (2011) Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 15: R173.
-
Krinsley JS, Grover A (2007) Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 35: 2262-2267.
-
Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM Jr, et al. (2008) Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg 74: 679-685.
-
Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, et al. (2010) Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med 38: 1021-1029.
-
Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, et al. (2008) Glucose variability and mortality in patients with sepsis. Crit Care Med 36: 2316-2321.
-
Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, et al. (2010) Glucose variability is associated with intensive care unit mortality. Crit Care Med 38: 838-842.
-
Krinsley JS (2009) Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol 3: 1292-1301.
-
Krinsley JS (2008) Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 36: 3008-3013.
-
Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, et al. (2009) The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care 13: R91.
-
Egi M, Bellomo R, Stachowski E, French CJ, Hart G (2006) Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 105: 244-252.
-
Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, et al. (2013) Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 17: R37.
-
Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R (2012) The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care 15: 151-160.
-
Thomann R, Schütz P, Müller B, Thomke S, Schoenenberger R, et al. (2013) Evaluation of an algorithm for intensive subcutaneous insulin therapy in noncritically ill hospitalised patients with hyperglycaemia in a randomised controlled trial. Swiss Med Wkly 143.
-
Patel KL (2008) Impact of tight glucose control on postoperative infection rates and wound healing in cardiac surgery patients. J Wound Ostomy Cont Nurs 35: 397-404.
-
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY-S, Blair D, Foster D, et al. (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360: 1283-1297.
-
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, et al. (2009) American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 15: 353-369.
-
Inzucchi SE (2006) Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 355: 1903-1911.
-
Mendez CE, Mok K-T, Ata A, Tanenberg RJ, Calles-Escandon J, et al. (2013) Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care 36: 4091-4097.
-
Korytkowski MT (2013) In-patient management of diabetes: Controversies and guidelines. Indian J Endocrinol Metab 17: S630-635.
-
Umpierrez G, Maynard G (2006) Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? J Hosp Med 1: 141-144.
-
Umpierrez GE, Palacio A, Smiley D (2007) Sliding scale insulin use: myth or insanity? Am J Med 120: 563-567.
-
Hirsch IB (2009) Sliding scale insulin--time to stop sliding. JAMA 301: 213-214.
-
Albrich WC, Rüegger K, Dusemund F, Schuetz P, Arici B, et al. (2013) Biomarker-enhanced triage in respiratory infections: a proof-of-concept feasibility trial. Eur Respir J 42: 1064-1075.
-
Schuetz P, Hausfater P, Amin D, Haubitz S, Faessler L, et al. (2015) Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care Lond Engl 19:377.
-
Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, et al. (2012) Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in "real life": an international, multicenter poststudy survey (ProREAL). Arch Intern Med 172:715-722.
-
Widmer D, Drozdov D, Rüegger K, Litke A, Arici B, et al. (2014) Effectiveness of Proadrenomedullin Enhanced CURB65 Score Algorithm in Patients with Community-Acquired Pneumonia in "Real Life", an Observational Quality Control Survey. J Clin Med 3: 267-279.
-
Conca A, Bossart R, Regez K, Schild U, Wallimann G, Schweingruber R, et al. (2012) OPTIMA - Optimierter Patienten-Transfer durch innovatives multidisziplinäres Assessment - Projektbeschreibung der Phase I. PrInterNet- Z Für Pflegewissenschaft 14:291-298.
-
Steiner D, Renetseder F, Kutz A, Haubitz S, Faessler L, et al. (2016) Performance of the Manchester Triage System in Adult Medical Emergency Patients: A Prospective Cohort Study. J Emerg Med 50: 678-689.
-
Swanson CM, Potter DJ, Kongable GL, Cook CB (2011) Update on inpatient glycemic control in hospitals in the United States. Endocr Pract 17: 853-861.





