International Journal of Diabetes and Clinical Research
The Safety, Efficacy and Treatment Satisfaction Comparison of Unchanged Premixed Insulin Regimen Plus Sitagliptin with Switch from the Premixed Insulin to Once-Daily Basal Insulin Plus Sitagliptin in Patients with Inadequately Controlled Type 2 Diabetes with Twice-Daily Premixed Insulin
Kanako Ono1,9, Akinobu Nakamura1, Junko Kawaguchi1, Masahiro Takihata1, Yuichiro Inoue1, Jun Shirakawa1, Akiko Kameda1, Yu Togashi1, Tsutomu Hayashi2, Takayuki Furuki3, Shun Ito4, Tatsuro Takano5, Satsuki Kawasaki6, Hiroshi Takeda7, Tetsuji Kaneko8,9, Mari Kimura1, Shunsaku Mizushima9 and Yasuo Terauchi1*
1Department of Endocrinology and Metabolism, Yokohama City University School of Medicine, Kanagawa, Japan
2Hayashi Diabetes Clinic, Kanagawa, Japan
3Minamiguchi Clinic in Kuzuhadai Hospital, Kanagawa, Japan
4Japanese Red Cross Tsukui Hospital, Kanagawa, Japan
5Fujisawa City Hospital, Kanagawa, Japan
6Shonan Fujisawa Tokushukai Hospital, Kanagawa, Japan
7Takeda Clinic, Kanagawa, Japan
8Department of Research Support and Coordination, Yokohama City University School of Medicine, Kanagawa, Japan
9Department of Public Health, Yokohama City University School of Medicine, Kanagawa, Japan
*Corresponding author:
Yasuo Terauchi, Department of Endocrinology and Metabolism, Yokohama City University School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama, Kanagawa, 236-0004, Japan, Tel: +81-45-787-2639, Fax: +81-45-781-5379, E-mail: terauchi@yokohama-cu.ac.jp
Int J Diabetes Clin Res, IJDCR-2-044, (Volume 2, Issue 5), Original Article; ISSN: 2377-3634
Received: August 08, 2015 | Accepted: September 28, 2015 | Published: September 30, 2015
Citation: Ono K, Nakamura A, Kawaguchi J, Takihata M, Inoue Y, et al. (2015) The Safety, Efficacy and Treatment Satisfaction Comparison of Unchanged Premixed Insulin Regimen Plus Sitagliptin with Switch from the Premixed Insulin to Once-Daily Basal Insulin Plus Sitagliptin in Patients with Inadequately Controlled Type 2 Diabetes with Twice-Daily Premixed Insulin. Int J Diabetes Clin Res 2:044. 10.23937/2377-3634/1410044
Copyright: © 2015 Ono K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: While adding sitagliptin to insulin therapy for Type 2 Diabetes Mellitus (T2DM) showing poor response to therapy with premixed insulin alone, we compared the safety, efficacy and treatment satisfaction of continuing treatment with the premixed insulin versus switching from the premixed insulin to basal insulin therapy.
Methods: The study was an open-label, randomized controlled trial conducted at 7 institutions in Japan. The study participants were randomly assigned to one of two treatment groups: a group in which insulin therapy was continued with twice-daily administration of premixed insulins (premixed insulin group) and a group in which premixed insulin therapy was switched to basal insulin administration (basal insulin group). Sitagliptin 50 mg/day was administered to both groups for 16 weeks.
Results: Forty-four patients (including 26 men and 18 women) were enrolled for this study, and 43 patients were completed (basal insulin group: 22 patients; premixed insulin group: 21 patients). At 16 weeks, the mean change in the HbA1c level from the 0 week was -0.22 ± 0.88% in the basal insulin group and -0.60 ± 0.78% in the premixed insulin group (P = 0.143). Also, the change of total Treatment Satisfaction evaluated by Diabetes Treatment Satisfaction Questionnaire change version (DTSQc) in the basal insulin group was significantly greater than that in the premixed insulin group.
Conclusions: Our study revealed that switching from the premixed insulin to once-daily basal insulin plus sitagliptin improved the change of total treatment satisfaction in T2DM although the changes in the HbA1c levels did not differ between the two groups.
Keywords
Type 2 diabetes, Basal insulin, Premixed insulin, Sitagliptin, Diabetes treatment satisfaction questionnaire
Introduction
Premixed insulins contain a mixture of long-and short-acting insulins in a single preparation and are usually injected twice daily. Premixed insulins are the most frequently prescribed treatment in Japan for Type 2 Diabetes Mellitus (T2DM) [1]. However, scarce data are available to guide the subsequent-step therapeutic strategies for T2DM showing inadequate glycemic control with premixed insulins.
Sitagliptin is a novel oral antidiabetic agent that selectively inhibits dipeptidyl peptidase-4 (DPP-4), to increase the blood concentrations of active glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Several clinical trials have shown the efficacy of sitagliptin, administered either alone or in combination with other oral antidiabetic agents, in T2DM, and its association with a minimal risk of hypoglycemia [2]. Recently, a few studies have examined the effects of adding sitagliptin to insulin therapy [3,4]. Vilsboll et al. [3] showed in a 24-week study that the addition of sitagliptin to insulin therapy improved the glycemic control in T2DM [3]. In this report, similar reductions of the HbA1c were observed in patients stratified according to the insulin type (long-acting and intermediate-acting insulins or premixed insulins) [3]. Also, it has been reported that addition of sitagliptin to multiple daily insulin injections therapy in patients with uncontrolled Japanese T2DM improved glycemic control without weight gain or an increase in the incidence of hypoglycemia [4].
Therefore, the addition of sitagliptin to insulin therapy could be a next-step therapeutic strategy for patients showing inadequate glycemic control in response to premixed insulins. While considering addition of sitagliptin to insulin therapy, either of two approaches may be adopted in relation to continuation of the premixed insulin therapy: either continuing administration of the premixed insulin or switching from premixed to basal insulin therapy. However, no randomized trials comparing these two approaches have been published. In the present study, while adding sitagliptin to insulin therapy for T2DM showing inadequate glycemic control in response to therapy with premixed insulins alone, we investigated the safety, efficacy, and treatment satisfaction of continuing treatment with the premixed insulin versus switching from the premixed insulin to basal insulin therapy.
Methods
Design overview
The study was an open-label, randomized controlled trial conducted at 7 institutions in Japan. A total of 44 patients with inadequately controlled T2DM (HbA1c, 6.9%-10.4%) in response to twice-daily administration of premixed insulins (Insulin human 30/70 (Novolin 30R®; Novo-nordisk, Denmark), Insulin aspart 30/70 (NovoRapid 30 Mix®; Novo-nordisk, Denmark), Insulin lispro 25/75 (Humalog® Mix75/25™; Eli Lilly and Company, United States of America), Insulin lispro 50/50 (Humalog® Mix50/50™; Eli Lilly and Company, United States of America)) were randomly assigned at a 1:1 ratio to one of the two following groups: a group in which the premixed insulin administration was switched to once-daily basal insulin (insulin glargine (Lantus®; Sanofi-aventis, France) or insulin detemir (Levemir®; Novo-nordisk, Denmark)) administration while adding sitagliptin at 50 mg/day (basal insulin group) or a group in which the twice-daily administration of premixed insulin was continued while adding sitagliptin at 50 mg/day (premixed insulin group). Both groups were followed up for 16 weeks. The primary outcome measure was the difference in the changes of the HbA1c level from the baseline to the end of 16 weeks of treatment between the two groups.
Setting and participants
Recruitment of the patients for this study between December 2011 and March 2012 by the physicians at the participant institutions was based on the criteria listed below. The inclusion criteria were: men or women with T2DM who showed inadequate glycemic control (HbA1c, 6.9-10.4%) in response to treatment using twice-daily administration of premixed insulins. The following patients were excluded: (1) patients with renal insufficiency (serum creatinine > 2.0 mg/dL), (2) patients with severe liver dysfunction, (3) patients who had received two kinds of insulin preparations, (4) patients who were judged as being inappropriate candidates for inclusion in the study by the physicians in charge.
Randomization and interventions
All the patients were randomized to the basal insulin group or the premixed insulin group by the permuted block method using a central computer-based randomization, and were followed up for 16 weeks. Both groups received sitagliptin at the dose of 50 mg/day for 16 weeks. At 0 week, if the HbA1c was 6.9-8.3%, the daily dose of basal insulin was decreased to 80% of the previous dose; if the HbA1c level was 8.4-10.4%, the dose of basal insulin was decreased to 90-100% of the previous insulin dose. During this trial, no changes in the administration of either sitagliptin or other oral hypoglycemic agents were allowed except sulfonylurea. If the dose of sulfonylurea was exceeded of 2 mg of glimepiride or 1.25 mg of glibenclamide at 0 week, it was reduced to 2 mg of glimepiride or 1.25 mg of glibenclamide during the study. If the dose of sulfonylurea is glimepiride ≤ 2 mg or glibenclamide ≤ 1.25 mg at 0 week, it was not changed. To achieve the target fasting blood glucose level of 110-130 mg/dl or postprandial blood glucose level of 140-180 mg/dl, the insulin doses were adjusted by 0-4 U at every clinic visit by the physicians, based on the data obtained from self-monitoring blood glucose or measurements carried out in blood samples by each physician. Diet and exercise therapy during the study was controlled by each physician.
Outcomes and follow-up
The primary outcome measure was the change of the HbA1c level from the baseline to the end of 16 weeks of treatment between the two groups. The key secondary outcomes were the body weight, insulin dose, clinic blood pressure (CBP), lipid profile, side effects, and patient-reported outcomes. At 0 and 16 weeks after patient randomization, each patient's body weight and CBP were measured and blood samples were collected to measure the blood levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG) and HbA1c; then, the value of serum low-density lipoprotein cholesterol (LDL-C) was calculated using the following equation: LDL-C = TC- (TG/5 + HDL-C). Monitoring for adverse effects such as hypoglycemia was carried out during this trial. Hypoglycemia was defined as a blood glucose < 70 mg/dl or the presence of symptoms of hypoglycemia. Severe hypoglycemia was defined as hypoglycemia with coma.
Patient-reported outcomes were measured using two validated, self-administered questionnaires, the diabetes treatment satisfaction questionnaire status version (DTSQs) and diabetes treatment satisfaction questionnaire change version (DTSQc) [5], at 5 institutions. The remaining institutions were not included in the patient-reported outcome sub-study, because of non-availability of consent for the survey by the physicians. The DTSQs contain 8 items, responses for which are scored on 7-point scale, from +6 to 0. The questions pertain to the satisfaction level of the patients are at the given time-point. It must be noted here that ceiling effects are often seen with satisfaction measures, where maximum or close-to-maximum scores at the baseline provide little opportunity for registering any improvement in the level of satisfaction with the treatment or strategy. The DTSQc was designed to overcome such ceiling effects. This instrument contains the same eight items as the DTSQs, however, the questions in this instrument are related to how much the satisfaction level of the patients has changed since before the trial began. The individual questions on the DTSQc were scored on 7-point scales from +3 to -3. Six of the eight items in both the DTSQs and the DTSQc were: Current treatment, Convenience, Flexibility, Understanding, likelihood of recommending their present treatment (Recommend), and 'satisfaction to continue with their present treatment (Continue)'. The scores for these six items were summed as the 'Overall' treatment satisfaction score in the range of +36 to 0 in the DTSQs or +18 to -18 in the DTSQc, with higher scores denoting greater treatment satisfaction or increased treatment satisfaction. 'Perceived frequency of hyperglycemia (Hyperglycemia)' and 'Perceived frequency of hypoglycemia (Hypoglycemia)' were included as one item each in the DTSQs and the DTSQc. The scores for perceived frequency of hyperglycemia and hypoglycemia were rated on a scale of +6 ('most of the time') to 0 ('never to time') in the DTSQs or of +3 ('much more of the time now') to -3 ('much less of the time now') in the DTSQc. The DTSQs and DTSQc were administered at 16 weeks, such that the DTSQs measured satisfaction with treatment in the previous few weeks, while the DTSQc measured how satisfaction with treatment had changed compared with satisfaction with treatment used before the study began, i.e. before the addition of sitagliptin and, in one group, a change of insulin.
Statistical analysis
The baseline characteristics are descriptively summarized using means and standard deviations for continuous variables and frequencies for categorical variables. Continuous variables were compared using the unpaired t-test or Mann-Whitney U test, and categorical variables using the chi-square test or Fisher's exact test. The unpaired t-test was used to examine the outcome measures, that is, the difference in the mean change from the baseline to the end of 16 weeks of treatment between the two groups. The changes from the baseline to the end of 16 weeks of treatment in each group were analyzed using a paired t-test. Statistical significance was set at two-tailed values of p < 0.05. All analyses were performed using the SPSS version 20.0 (SPSS, Inc., Chicago, IL).
Results
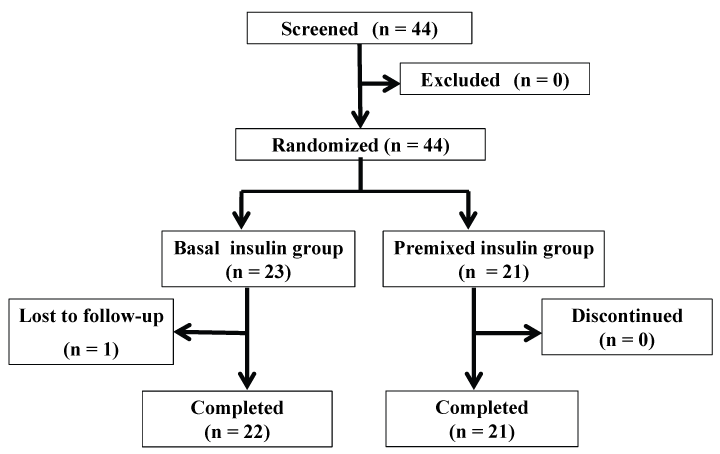
Forty-four patients (including 26 men and 18 women) with T2DM who showed inadequate glycemic control (HbA1c, 6.9-10.4%) in response to twice-daily premixed insulin therapy were enrolled for this study. Every patient who met the inclusion criteria prior to this trial was randomly assigned to the basal insulin group or the premixed insulin group. Of these 44 patients, the trial could be completed in 43 patients (basal insulin group: 22 patients; premixed insulin group: 21 patients). One patient from the basal insulin group was excluded from the analysis because she was lost-to-follow-up. The final follow-up rate was 97.7% (Figure 1).
.
Figure 1: Flow-chart of the study participants through the trial. Of the 44 patients who were enrolled, the trial could be completed in 43 (basal insulin group: 22 patients; premixed insulin group: 21 patients). One patient from the basal insulin group was excluded because she was lost to follow-up.
View Figure 1
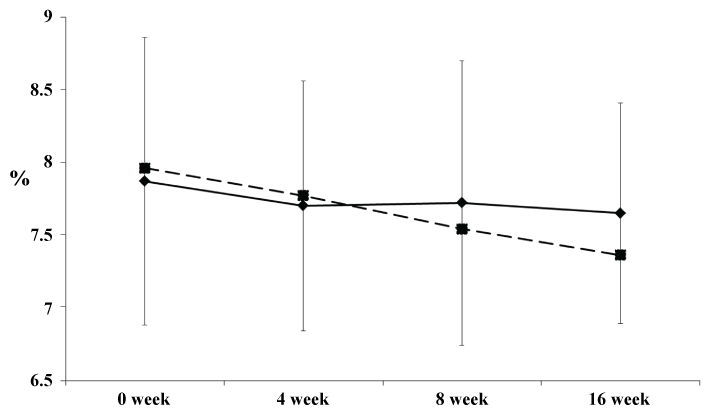
Table 1 shows the baseline characteristics of the 43 patients in whom this trial could be completed. No statistically significant differences in the baseline characteristics including diabetic complication were observed between the two groups (Supplemental table S1). The kind of the premix insulins used at baseline were insulin aspart 30/70 (NovoRapid 30 Mix®; Novo-nordisk, Denmark), insulin human 30/70 (Novolin 30R®; Novo-nordisk, Denmark), insulin lispro 25/75 (Humalog® Mix75/25™; Eli Lilly and Company, United States of America) and insulin lispro 50/50 (Humalog® Mix50/50™; Eli Lilly and Company, United States of America) (Supplemental table S2). Information on concomitant medications of oral hypoglycemic agents, anti-hypertensive agents and anti-dyslipidemic agents were shown in Supplemental table S3-S5. Table 2 shows the clinical parameters at 0 week and 16 weeks in the study groups. Table 3 shows the differences in the changes of the parameters from 0 week to the end of 16 weeks of treatment between two groups. In the basal insulin group, the dose of insulin was reduced from 24.8 ± 11.1 U/day to 19.6 ± 9.8 U/day (79.0%) at 0 week according to the protocol for changing premixed insulin to basal insulin. Therefore, the mean change of the insulin dose at 0 week in the basal insulin vs. premixed insulin group was -5.2 ± 3.4 U/day vs. -1.2 ± 2.1 U/day, respectively (P < 0.001), and the change of the insulin dose from 0-16 weeks was -0.09 ± 2.88 U/day vs. -0.38 ± 2.48 U day, respectively (Table 3). Thus, the dose of insulin remained essentially unchanged during the trial in both groups. The mean HbA1c level in the basal insulin group did not improve for 16 weeks. On the other hand, in the premixed insulin group, the HbA1c level significantly improved for 16 weeks (Figure 2). The changes in the HbA1c level from the baseline in the basal insulin group vs. premixed insulin group were -0.22 ± 0.88% vs. -0.60 ± 0.78% after 16 weeks (P = 0.143) of treatment.
.
Figure 2: Changes of the HbA1c levels from 0 to 16 weeks in the basal insulin group and premixed insulin group. Values are expressed as means ± S.D. Basal insulin group: diamonds; premixed insulin group: squares.
View Figure 2
![]()
Table 1: Baseline characteristics of the subjects in whom this trial was completed.
View Table 1
The mean body weight was significantly reduced for 16 weeks in the basal insulin group, but not in the premixed insulin group. The mean change in the body weight from 0 week to the end of 16 weeks of treatment was -0.68 ± 1.03 kg in the basal insulin group and. +0.38 ± 1.72 kg in the premixed insulin group (P = 0.019). The mean diastolic CBP was significantly decreased for 16 weeks both in the basal insulin group and the premixed insulin group. The mean change in the diastolic CBP from 0 week to 16 weeks was -5.67 ± 8.84 mmHg in the basal insulin group and -5.40 ± 8.76 mmHg in the premixed insulin group (P = 0.923). However, no significant changes of the systolic CBP or the serum levels of TC, TG, HDL-C and LDL-C were observed in either group.
![]()
Table 2: Time courses of changes in the clinical parameters in the basal insulin group (A) and in the premixed insulin group (B).
View Table 2
![]()
Table 3: Comparison of the changes in clinical parameters from 0-16 weeks between the two groups.
View Table 3
Hypoglycemia events were observed during the period from 0 week to the end of 16 weeks of treatment in 2 patients (9.1%) from the basal insulin group and 2 patients (9.5%) from the premixed insulin group. Also, itching was observed in one patient from the premixed insulin group. No cases of severe hypoglycemia, rash or gastrointestinal symptoms were encountered in either group during the period of this trial.
Patient-reported outcome measures were assessed in a total of 33 patients (including 17 patients of the basal insulin group and 16 patients in the premixed insulin group) from 5 institutions. The 'Overall' Satisfaction score for the DTSQs at post-trial was 25.88 ± 8.71 in the basal insulin group and 22.13 ± 4.54 in the premixed insulin group, with no statistically significant difference between the two groups. In regard to the four items of 'Current treatment', 'Understanding', 'Recommend' and 'Continue' in the DTSQs survey conducted at the end of the treatment, there were no statistically significant differences in the scores between the two groups, although these scores tended to be higher in the basal insulin group than those in the premixed insulin group. The scores for the two items of 'Convenience' and 'Flexibility' were significantly higher in the basal insulin group than those in the premixed insulin group (Table 4A). On the other hand, the DTSQc, which measured the change in satisfaction from baseline in a single score, confirmed that the 'Overall' score of treatment satisfaction was significantly higher in the basal insulin group (mean score 10.53 ± 6.73) than that in the premixed insulin group (mean score 4.13 ± 4.64, P = 0.004), and that the proportion of 'satisfied' patients (defined as an 'Overall' satisfaction score of > 6) was 81.3% in the basal insulin group and 33.3% in the premixed insulin group (P = 0.007). Individual results for the six Satisfaction items of the DTSQc showed significantly greater improvements in all items with the basal insulin group than with the premixed insulin group, except the scores for the items of 'Current treatment' and 'Understanding,' which were indistinguishable between the basal insulin group and the premixed insulin group (Table 4B). No statistically significant differences in the frequencies of hypoglycemia and hyperglycemia were observed between the two groups as judged from the DTSQs and DTSQc surveys conducted at the end of the treatment.
![]()
Table 4: The scores of the individual DTSQs (A) and DTSQc (B) at post-trial.
View Table 4
Discussion
Since twice-daily premixed insulin therapy is insufficient to maintain adequate glycemic control in many T2DM [6], it is important to establish the next-step therapeutic strategy for patients showing inadequate glycemic control in response to premixed insulin therapy. In this study, while adding sitagliptin to insulin therapy for T2DM showing poor response to therapy with premixed insulins alone, we compared the safety and efficacy of continuing the premixed insulin administration versus switching from premixed to basal insulin therapy, in an attempt to evaluate which method might be preferable for such patients. Thus, this trial was designed to imitate one of the most frequently encountered clinical situations by physicians in daily practice.
The results of this study showed that there was no significant difference in the mean change of the HbA1c level from 0 week to the end of 16 weeks of treatment between the basal insulin group and the premixed insulin group. Also, there were no significant differences in the mean changes of the HbA1c level from 0 week to 4, 8, and 12 weeks of treatment between the two groups. However, significant improvement of the HbA1c level was observed after 16 weeks of treatment in the premixed insulin group, while only a tendency towards improvement during this period was observed in the basal insulin group. Vilsboll et al. [3] reported that the addition of sitagliptin 100 mg significantly reduced the HbA1c by 0.6% at the end of 24 weeks treatment as compared to administration of placebo (0.0%), regardless of the insulin treatment protocol [3]. Our results in respect of the efficacy of addition of sitagliptin were basically consistent with this report, even though there were differences in the baseline characteristics and dose of sitagliptin between this report and our study.
In previous reports, Hammer, et al. [7] have shown that, in T2DM inadequately controlled with premixed insulin, switching therapy to basal insulin plus oral antidiabetic drugs resulted in a significant decrease of the HbA1c level by approximately 1% [7]. Shigihara et al. [8] also reported that switching from twice-daily premixed insulin administration to once-daily basal insulin therapy supported by oral agents, such as sulfonylureas, significantly improved the HbA1c level from 8.3% to 7.7% after 24 weeks of treatment in Japanese type 2 diabetic patients [8].
The problem in this study is that the addition of sitagliptin produced no significant decrease of the HbA1c levels in the basal insulin group. One possible reason may pertain to the study design, in that the insulin dose was decreased by up to 20% at 0 week in the basal insulin group; at 0 week, the dose of insulin was reduced from 24.8 ± 11.1 U/day to 19.6 ± 9.8 U/day (79.0%) in the basal insulin group, and thereafter the dose remained essentially unchanged until after 16 weeks. In Shigihara's study, the dose of insulin was decreased to 70% of the previous insulin dose while switching from twice-daily premixed insulin therapy to basal insulin therapy [8]. Notwithstanding, the HbA1c level decreased significantly in this group. Since sitagliptin, unlike sulfonylurea, substantially reduces the postprandial blood glucose excursions [9], we suggest that decrease of the insulin dose could lead to an inadequate dose of basal insulin, resulting in the absence of any significant decrease of the HbA1c level in the basal insulin group despite the addition of sitagliptin. Yang et al. [10] suggest that better glucose control with a good safety and tolerability profile could be achieved with a daily dose of insulin glargine optimized to about 0.4 U/kg body weight in Chinese patients showing unsatisfactory glycemic control in response to premixed insulin therapy [10]. According to a large-scale investigation in Japan, the HbA1c and mean insulin dose in patients with type 2 diabetes treated by diabetes specialists prescribing insulin plus oral antidiabetic agents were 7.7% and 29.5 U, respectively [1]. In our study, the insulin dose for the basal insulin group was 24.8 U (0.41 U/kg) prior to the start of the study, therefore, there might have been no need to decrease the dose of insulin when switching from premixed insulin to basal insulin. Thus, further trials with the study design focused on the degree of dose reduction are needed for correct evaluations in the basal insulin group.
Interestingly, at the end of 16 weeks of treatment, the mean changes in the body weight from the 0 week value in the basal insulin group were significantly decreased as compared with those in the premixed insulin group (Table 3). Since the body weight of the subjects was stable even after the addition of sitagliptin [11], we suggest that the decrease of the insulin doses in the basal insulin group may have had an influence on these body weight changes. Additionally, there was no difference in the frequency of hypoglycemia or severe hypoglycemia between the two groups. In regard to this point also, further studies with the study design focused on the degree of dose reduction that would be associated with an increase in the frequency of hypoglycemia in the basal insulin group are needed.
In this study, the diastolic CBP decreased significantly after 16 weeks of treatment in both groups. We suggest that this reduction of the diastolic CBP reduction was attributable, at least in part, to the addition of sitagliptin. Sitagliptin is reportedly associated with small, but significant reductions of the 24-hour ambulatory systolic and diastolic blood pressure as compared to placebo in hypertensive patients without diabetes [12]. Body weight loss seems to be closely involved in the mechanism underlying this antihypertensive effect [13]. In our study, however, there was no change of the body weight from the baseline in the premixed insulin group despite the CBP reduction, implying that the antihypertensive effect of sitagliptin was not related to the body weight loss. Kubota et al. [14] reported the pleiotropic effects of sitagliptin in a retrospective observational study of 940 Japanese type 2 diabetic patients [14]. In this study, as compared with that at the start of treatment, significant decrease of the blood pressure was seen at the end of 12 weeks of treatment. The decrease of the CBP in patients receiving sitagliptin may be related to the sodium-diuretic action of the drug [14].
The results of the DTSQc evaluation showed that the patient satisfaction level with the treatment was significantly improved in the basal insulin group as compared with that in the premixed insulin group. Yang et al. [10] reported significantly improved patient satisfaction following a treatment switch from premixed insulin therapy to once-daily insulin glargine therapy [10]. A number of factors may contribute to the better satisfaction level of the patients with basal insulin: for example, the once-daily dosing of basal insulin offers improved convenience as compared to the twice-daily dosing required in premixed insulin therapy, and the improved flexibility of basal insulin in that the daily dosing can be administered independently of the time of day or meals, as long as it is administered approximately every 24 h. Data derived from a quality of life questionnaire survey have emphasized the important contribution of dietary freedom to the overall quality of life [15]. In addition, with the satisfaction associated with the weight loss in the basal insulin group, the patients' treatment adherence may improve. Thus, these patient reported outcome data may make an important contribution to clinical decisions [16].
The present study had several limitations, such as the relatively small number of participants and the limited follow-up period. Also, in regard to hypoglycemia, missing asymptomatic hypoglycemia might exist. More importantly, the decrease in the dose of basal insulin at 0 week may have contributed to the insufficient glycemic control in the basal insulin group despite the addition of sitagliptin. Nevertheless, these findings may reflect the effects of addition of sitagliptin to insulin therapy in actual clinical practice. As regards the results of the DTSQ, DTSQ was obtained only in a subgroup of the study. When we compared the baseline characteristics of the subjects performed DTSQ (Supplemental tables S6-S8), the basal insulin group showed younger age and shorter duration of the disease compared with the premixed insulin group, which might contribute to better outcome of the basal insulin group.
In conclusion, switching from the premixed insulin to once-daily basal insulin plus sitagliptin improved the change of total treatment satisfaction in patients with T2DM although the changes in the HbA1c levels did not differ between the two groups.
Competing Interests
Yasuo Terauchi received honoraria for lectures from MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Novartis Pharma K.K.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Kowa Pharmaceutical Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; and AstraZeneca K.K. and obtained research support from MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Novartis Pharma K.K.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Dainippon Sumitomo Pharma Co., Ltd.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; Astellas Pharma, Inc.; Pfizer Japan, Inc.; and AstraZeneca K.K. The other authors have no potential conflicts of interest to declare in relation to the publication of this article.
Authors' Contributions
All authors except TK and SM treated the patient. KO, AN and YT drafted and revised the manuscript. TK and SM were involved in the statistics management. All authors have read and approved the final manuscript.
Acknowledgments
The authors are deeply grateful to Prof. Clare Bradley, Jonathan Gilbride and Alison Wilson for providing helpful comments and suggestions on the DTSQ. This work was supported in part by Grants in Aid for Scientific Research (B) 21390282 and (B) 24390235 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, a Grant for the Strategic Japanese-Danish Cooperative Program on Molecular Diabetology from the Japan Science and Technology Agency, a Grant in Aid from the Uehara Memorial Foundation (to Y.T.).
Ethical Statement
The Declaration of Helsinki compliant study protocol by the Ethics Committee of Yokohama City University School of Medicine (Yokohama, Japan) was approved in all of Yokohama City University School of Medicine and the 6 affiliated hospitals. All the subjects provided their written informed consent for participation in the study.
Trial registration: UMIN (http://www.umin.ac.jp/) 000008884
References
-
Arai K, Takai M, Hirao K, Matsuba I, Matoba K, et al. (2012) Present status of insulin therapy for type 2 diabetes treated by general practitioners and diabetes specialists in Japan: Third report of a cross-sectional survey of 15,652 patients. J Diabetes Invest 3: 396-401.
-
Gerrald KR, Van Scoyoc E, Wines RC, Runge T, Jonas DE (2012) Saxagliptin and sitagliptin in adult patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 14: 481-492.
-
Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, et al. (2010) Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 12: 167-177.
-
Shimoda S, Iwashita S, Ichimori S, Matsuo Y, Goto R, et al. (2013) Efficacy and safety of sitagliptin as add-on therapy on glycemic control and blood glucose fluctuation in Japanese type 2 diabetes subjects ongoing with multiple daily insulin injections therapy. Endocr J 60: 1207-1214.
-
Bradley C (1999) Diabetes treatment satisfaction questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care 22: 530-532.
-
Fritsche A, Larbig M, Owens D, Haring HU, GINGER study group (2010) Comparison between a basal-bolus and a premixed insulin regimen in individuals with type2 diabetes-results of the GINGER study. Diabetes Obes Metab 12: 115-123.
-
Hammer H, Klinge A (2007) Patients with type 2 diabetes inadequately controlled on premixed insulin: effect of initiating insulin glargine plus oral antidiabetic agents on glycaemic control in daily practice. Int J Clin Pract 61: 2009-2018.
-
Shigihara N, Tamaki M, Goto H, Kawai J, Fujitani Y, et al. (2010) Efficacy and safety of switching from premix twice daily injection to sulfonylurea and once daily insulin glargine in Japanese type 2 diabetes (JUN-LAN Study 8). J Japan Diab Soc 53: 157-161.
-
Arnolds S, Dellweg S, Clair J, Dain MP, Nauck MA, et al. (2010) Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care 33: 1509-1515.
-
Yang W, Lv X, Li Q, Jia W, Tian H (2012) A prospective study to optimize insulin treatment by switching to insulin glargine in type 2 diabetic patients previously uncontrolled on premixed insulin: the optimization study. Curr Med Res Opin 28: 533-541.
-
Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, et al. (2008) Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 79: 291-298.
-
Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, et al. (2008) Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 48: 592-598.
-
Horton ES, Silberman C, Davis KL, Berria R (2010) Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 33: 1759-1765.
-
Kubota A, Maeda H, Kanamori A, Matoba K, Jin Y, et al. (2012) Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res 4: 309-313.
-
Sundaram M, Kavookjian J, Patrick JH, Miller LA, Madhavan SS, et al. (2007) Quality of life, health status and clinical outcomes in Type 2 diabetes patients. Qual Life Res 16: 165-177.
-
Patient-reported outcomes are superior in patients with Type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: results from a randomized, open-label study. Diabet Med 28: 715-723.