International Journal of Cancer and Clinical Research
Metronomic Chemotherapy Preserves Quality of Life Ensuring Efficacy in Elderly Advanced Non Small Cell Lung Cancer Patients
Francesca De Iuliis1, Stefania Vendittozzi2, Ludovica Taglieri1, Gerardo Salerno1, Rosina Lanza3 and Susanna Scarpa1*
1Experimental Medicine Department, University Sapienza, Italy
2Radiology-Oncology and Anatomo-Pathology Department, University Sapienza, Italy
3Gynecology and Obstetrics Department, University Sapienza, Italy
*Corresponding author:
Susanna Scarpa, Dipartimento Medicina Sperimentale, Viale Regina Elena 324, Università Sapienza, 00161 Roma, Italy, Tel: +39-3395883081, E-mail: susanna.scarpa@uniroma1.it
Int J Cancer Clin Res, IJCCR-3-046, (Volume 3, Issue 2), Original Article; ISSN: 2378-3419
Received: January 28, 2016 | Accepted: February 25, 2016 | Published: March 01, 2016
Citation: Iuliis FD, Vendittozzi S, Taglieri L, Salerno G, Lanza R, et al. (2016) Metronomic Chemotherapy Preserves Quality of Life Ensuring Efficacy in Elderly Advanced Non Small Cell Lung Cancer Patients. Int J Cancer Clin Res 3:046. 10.23937/2378-3419/3/2/1046
Copyright: © 2016 Iuliis FD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Metastatic non small cell lung cancers (NSCLC) are diseases with poor prognosis and platinum-based doublet chemotherapy still remains their standard cure. Elderly patients often present comorbidities that limit the utilization of this chemotherapy; therefore these patients should have a first-line treatment with low toxicity and capable to preserve the quality of life (QoL) but, at the same time, to ensure the best possible response. Furthermore, a first-line treatment allows patients to be fit for further treatments, prolonging overall survival. At this regard, metronomic chemotherapy can be an optimal choice for elderly, able to improve QoL and to obtain an optimal palliation. We retrospectively studied a cohort of 41 elderly advanced NSCLC patients with different histotypes, treated with metronomic chemotherapy (weekly carbo-paclitaxel or vinorelbine as single agent) as first choice and we analyzed the tolerability, the impact on QoL and the efficacy of these schedules: no toxicity of 3 and 4 grade was observed, together to a clinical benefit of 43%. We administered FACT-L test to evaluate QoL at baseline and after 4 months and found a significant improvement in all FACT-L parameters: physical, social, emotional and functional, confirming a QoL improvement. At a median follow-up of 20.2 months the progression free survival was of 6 months and the overall survival was of 15 months. These results suggest that metronomic chemotherapy can be an effective choice of treatment for elderly NSCLC patients and further trials with more patients are needed to confirm this proposal.
Keywords
Elderly, Carboplatin, Paclitaxel, Vinorelbine, Non-small cell lung cancer, Quality of life
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with a five years survival rate of about 15%; approximately 85% of all cases are non small cell lung cancers (NSCLC) [1]. The identification of specific mutations has led in the last ten years to the development of molecular targeted agents, which have improved the overall survival of metastatic patients [2]. Unfortunately, not all types of lung tumor express the specific mutations targets of biological agents, so chemotherapy is the only choice of treatment for these cases. Often elderly patients with advanced NSCLC have comorbidities, which prevent the administration of standard schedules of chemotherapy.
A new modality of chemotherapy administration is the metronomic schedule, based on the regular frequent use of lower doses of conventional drugs, proposed as an emerging alternative to conventional chemotherapy. Metronomic chemotherapy is sometimes more effective than the classic schedule, due to the continuous presence of lower doses of the drug in the patient; and it is also more tolerable, since the higher dose used in the classis schedule often limits the patient capacity to handle the side effects [3]. Furthermore, the cytostatic continuous action of metronomic administration can overcome drug resistance, assuring efficacy without great toxicity [3]. Carboplatin, paclitaxel and vinorelbine are the most utilized agents for lung cancer treatment, and the same agents are used also in metronomic schedules [4].
Quality of life (QoL) is an important prognostic factor in all cancer patients, especially in lung cancer [5]; patients with poor performance status (PS) aren’t fit for treatments, and they are candidates for palliative care only. Therefore, it is important to offer to these patients a first-line treatment that preserves QoL, limits toxicity, ensures the best possible response and allows patients to be fit for further treatments prolonging their overall survival (OS).
In our experience we have chosen to treat 41 elderly patients with advanced NSCLC with metronomic chemotherapy in first line, independently from their tumor histology, with the principal aim to preserve their QoL by reducing therapy toxicity. We have demonstrated, beyond a perfect adherence with the therapy and no grade 3 and 4 toxicities, a relevant clinical benefit for our cohort of patients, with an efficacy not inferior to that of traditional regimens, in terms of disease free survival (DFS) and OS.
Patients and Methods
We have retrospectively analyzed a cohort of 41 advanced NSCLC patients treated in our institution between 2009 and 2013. All 41 patients had inoperable NSCLC (clinical stage assigned on the basis of the Seventh Edition of the TNM Classification for Lung Cancer) and median age 75 years (range 70-83), 30 males and 11 females. Eligibility criteria were the following: histologically measurable NSCLC with at least one measurable lesion according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria, minimal life expectancy of 16 weeks, serum creatinine within normal limits (creatinine clearance > 30 ml/min by the Cockroft and Gault formula), hemoglobin ≥ 10 g/L, white blood cell conts ≥ 3.5 × 109/L, absolute neutrophil count (ANC) ≥ 1.5 × 109/L, platelets ≥ 150 × 109/L, total serum bilirubin ≤ 1.5 × upper normal limit (UNL), transaminases ≤ 2.0 × upper normal limit (UNL) unless attributed to liver metastases. All patients carried out a total body computed tomography (CT) scan at baseline and every three cycles to assess tumor response. Tumor response and disease progression were assessed according to RECIST criteria. 25 patients were treated with weekly carboplatin AUC2 in association to paclitaxel (80 mg/mq weekly) d 1, 8,15 q28 for 3-4 courses and 16 patients with vinorelbine 50 mg total dose d 1-3-5 q7 until diseae progression or unacceptable toxicity. Only 5 adenocarcinoma patients had epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKi) administered before metronomic chemotherapy.
Toxicity and QoL were evaluated as primary endpoints: the first was graded according to the National Cancer Institute (NCI) - Common Toxicity Criteria for AE (version 3.0); the second was evaluated by administering version 2 Functional Assessment of Cancer Therapy - Lung (FACT-L) test to our patients [6-8]. FACT-L test is a 44-item questionnaire that measures multidimensional quality of life; all questions are rated on five-points ranging from 0 (negative) to 4 (highly positive). Descriptive statistics was used to determine the overall QoL for patients at baseline and at 4 months. Matched t tests were conducted to discern whether the quality-of-life indicator (p < 0.05 was considered significant) differed from baseline to 4 months. Statistical analysis was performed using Graph Pad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Overall response rate (ORR), clinical benefit (CB), progression free survival (PFS) and overall survival (OS) were evaluated as secondary endpoints. The ORR was reported with its 95% confidence interval. OS and PFS were calculated by the Kaplan-Meier product-limit method. Statistical evaluation was performed by means of the statistical software CRAN 2.4.0.
Results
The clinical characteristics of all 41 patients were collected and listed (Table 1): 23 squamous cell carcinoma, 3 large cell carcinoma and 15 adenocarcinoma. No treatment-related death occurred. None of the patients required G-CSF (granulocyte-colony stimulating factors) for neutropenia or erythropoietin for anemia. Treatment was well tolerated, without any delay or dose reduction.
![]()
Table 1: Description of metastatic NSCLC patients. ADC (adenocarcinoma); SCC (squamous cell carcinoma); LCC (large cell carcinoma); KPS (Karnofsky performance status); SRS (stereotactic radiosurgery); WBR (whole brain radiotherapy); RT (radiotherapy).
View Table 1
Toxicity was evaluated and resulted minimal in all patients and no adverse events of grade (G) 3 and 4 were observed, only G1 and G2 toxicity was detected (Table 2). The following adverse events, classified as G1, occurred: neutropenia in 10 patients (24.39%), anemia in 20 patients (48.78%), neuropathy in 30 patients (73.17%), asthenia in 15 patients (36.58%) and alopecia in 39 patients (95.12%). The G2 adverse events were: neutropenia in 5 patients (13.19%), anemia in 10 patients (24.39%), asthenia in 10 patients (24.39%) and neuropathy in 5 patients (12.19%). No patients suffered for thrombocytopenia, mucositis, nausea or vomiting neither for febrile neutropenia. The quality of life of all patients was analyzed by FACT-L test at baseline and at 4 months (Table 3) and a significant modification was found in all parameters: physical well-being (p = 0.0002), social/family well-being (p = 0.001), emotional well-being (p = 0.002) and functional well-being (p = 0.001), demonstrating a significant improvement in QoL from baseline to 4 months.
![]()
Table 2: Description of patient toxicity.
View Table 2
The average length of the treatment was 3 months, while the median response lenght was 6 months (95% CI, 5.2-7.8). One patient treated with carbo-paclitaxel achieved complete response (2.5%), and 25 patients (12 treated with carbo-paclitaxel and 13 with vinorelbine) achieved partial responses (61%), 15 patients (37%) had stable disease. Only the patients who did not progress beyond six months have been considered for the clinical benefit, and resulted that 18 patients (44%) received a clinical benefit (Table 4). Overall response rate was 63.5% (95% CI, 58.1-72), and it was achieved in 26 patients including 1 complete response (2.5%) and 25 partial responses (61%), while 15 patients (37%) had stable disease and no patients had progression of the disease. Clinical benefit, observed in 18 patients, was 44% (95% CI, 36.2-51.5) (Table 4).
![]()
Table 3: Data of quality of life by FACT-L test. SD: standard deviation.
View Table 3
![]()
Table 4: Clinical benefit (CB) of the patients (CR + PR + SD ≥ 6 months). CR: complete response; PR: partial response; SD: stable disease.
View Table 4
Thirty five patients died for tumor progression (85.4%), five (12.2%) died due to causes not connected with tumors, while one patient is still alive. At a median follow-up of 20.2 months (range 12.3-32.2) the overall survival (OS) was 15 months (95% CI, 14-17) and the progression free survival (PFS) was 6 months (95% CI, 5-9) (Figure 1).
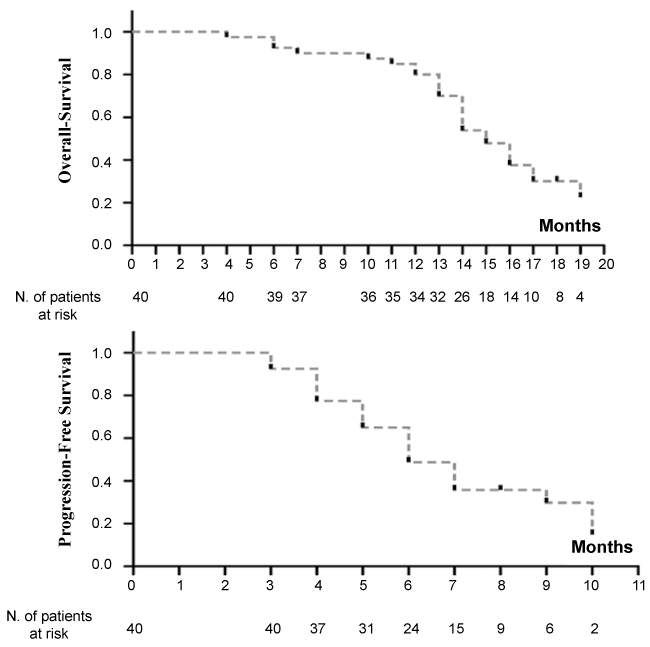
.
Figure 1: Kaplan-Meier curves of overall survival and of progression free survival in our cohort of metastatic NSCLC patients.
View Figure 1
Discussion
We have retrospectively selected a heterogeneous cohort of elderly patients with oligometastatic and multimetastatic NSCLC characterized by a performance status of 1/2, and demonstrated that metronomic schedule was more tolerable in these patients preserving their quality of life. At the same time, metronomic therapy was not inferior in terms of efficacy (evaluating DFS and OS) to the classic schedules.
Often elderly NSCLC patients are unfit for chemotherapy and a consensus on the best option for them doesn’t exist. Weekly carbo-paclitaxel is a valid option for this kind of patients [9,10], while metronomic vinorelbine as single agent is an optimal choice of treatment for patients unsuitable for platinum in first line [11]. The majority of metronomic schedules in lung cancer are performed with platinum agents in association with taxanes. A recent study of 167 patients (median age 65 years) compared the standard tri-weekly to the weekly schedule of carboplatin and paclitaxel in advanced NSCLC patients: toxicity was significantly decreased in the metronomic (weekly) arm, and for the first time this kind of administration has shown a better response than the tri-weekly standard schedule [12]. The Four Arms Comparative Study analyzed four standard platinum based regimens (with irinotecan, paclitaxel, gemcitabine or vinorelbine) to define the most effective schedule in first line, and the results demonstrated a similar efficacy among all these schedules [13]. In another study, AUC6 carboplatin was administered every 21 days with weekly paclitaxel (at the dose of 90 mg/mq) in a cohort of elderly patients, showing an higher efficacy respect the arm treated with monotherapy, but with an elevated incidence of febrile neutropenia [14].
We think that carbo-paclitaxel in a weekly administration can be a feasible and effective option for elderly, also in the presence of aggressive metastatic disease, all studies in fact conclude that the weekly regimen gives an advantage in toxicity, but not all of these studies demonstrate a comparable advantage in survival respect to the three weeks schedule [15]. We have chosen the minimal effective dose, based on metronomic theory, in order to minimize toxicity and to preserve the quality of life. The treatment with vinorelbine showed an efficacy comparable to carbo-paclitaxel in our cohort of patients. The maintenace of a costant lower dose of chemoterapeutics with a weekly schedule may be important in determining disease reduction and inducing less side effects. We found that no patient had G3 or G4 toxicity, with an optimal adherence to the treatment and no interruptions of the schedule; all the parameters of FACT-L test demonstrated a gain from baseline in terms of physical, social, emotional and functional well being.
The two prognostic factors that mainly influence long-term survival are the objective response after chemotherapy and the performance status [16]. In our cohort, although all patients had a performance status of 1/2, the overall response rate was 63.5% and the quality of life improved respect to baseline due to chemotherapy. The reason of the optimal efficacy of this weekly schedule is based on metronomic theory, whereby tumor response (or stable disease) is obtained when low dose of chemotherapy is administered with no intervals or in a short range of intervals [17]. The efficacy of metronomic therapy has been previously demonstrated by our group and by other authors in other settings of patients and in other kind of cancers, especially for elderly people [18,19]. Metronomic chemotherapy didn’t cause G3-4 neutropenia in our cohort of patients, contributing to preserve the quality of life, yet neutropenia chemotherapy-related is a prognostic factor for survival in patients with advanced NSCLC [1].
From our experience, we can suggest that first line metronomic chemotherapy is an effective and less toxic alternative for every elderly NSCLC patient, preserving the quality of life, as demonstrated by the score of FACT-L test. These schedules, constituted by weekly carbo-paclitaxel and vinorelbine as single agent, are characterized by the lack of toxicity with no G3 and G4 side effects. The benefit has been demonstrated, although only in 41 patients with lung cancer, either for squamous cell cancer than for adenocarcinoma. Due to the small cohort of our patients and to the retrospective nature of our study, it should be necessary to perform greater prospective studies in which metronomic carbo-paclitaxel and vinorelbine as single agent at the used dosages are compared to the standard three weeks schedule, in order to find out whether our data can be confirmed.
Conclusions
First line metronomic chemotherapy is an effective and less toxic alternative for every elderly non small cell lung cancer patient, since this therapy preserves the quality of life and gives the same benefits as the classic schedule. Furthermore, metronomic chemotherapy is characterized by a significant decrease of toxicity and less side effects as compared to classic therapy.
Abbreviation Field
NSCLC: non small cell lung cancers; QoL: quality of life; PS: performance status; OS: overall survival; DFS: disease free survival; RECIST: response evaluation criteria in solid tumors; AUC: area under curve; ANC: absolute neutrophil count; UNL: upper normal limit; CT: computed tomography; EGFR: epidermal growth factor receptor; TKi: tyrosine kinase inhibitors; NCI: National Cancer Institute; FACT-L: functional assessment of cancer therapy – lung; ORR: overall response rate; CB: clinical benefit; PFS: progression free survival; G-CSF: granulocyte-colony stimulating factors; G: grade; CI: confidence interval.
Ethical Statement
All authors declare that they have no competing interests. All patients signed an informed consent.
References
-
De Marinis F, Bria E, Baas P, Tiseo M, Camerini A, et al. (2015) Treatment of Unfit Patients With Advanced Non-Small-Cell Lung Cancer: Definition Criteria According an Expert Panel. Clin Lung Cancer 16: 399-405.
-
Oxnard GR, Binder A, Jänne PA (2013) New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 31: 1097-1104.
-
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4: 423-436.
-
Briasoulis E, Aravantinos G, Kouvatseas G, Pappas P, Biziota E, et al. (2013) Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. BMC Cancer 13: 263.
-
Maione P, Perrone F, gallo C, Manzione L, Piantedosi F, et al. (2005) Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multi-center Italian lung cancer in the elderly study. J Clin Oncol 23: 6865-6872.
-
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, et al. (1995) Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12: 199-220.
-
Browning KK, Ferketich AK, Otterson GA, Reynolds NR, Wewers ME (2009) A psychometric analysis of quality of life tools in lung cancer patients who smoke. Lung Cancer 66: 134-139.
-
Juliana F, Jardim JR, Fernandes AL, Jamnik S, Santoro IL (2010) Reliability of the Brazilian version of the Functional Assessment of Cancer Therapy-Lung (FACT-L) and the FACT-Lung Symptom Index (FLSI). Clinics (Sao Paulo) 65: 1247-1251.
-
Gridelli C, Balducci L, Ciardiello F, Di Maio M, Felip E, et al. (2015) Treatment of Elderly Patients With Non-Small-Cell Lung Cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 16: 325-333.
-
Camerini A, Puccetti C, Donati S, Valsuani C, Petrella MC, et al. (2015) Metronomic oral vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer: results of a phase II trial (MOVE trial). BMC Cancer 15: 359.
-
Meoni G, Cecere FL, Lucherini E, Di Costanzo F (2013) Medical treatment of advanced non-small cell lung cancer in elderly patients: a review of the role of chemotherapy and targeted agents. J Geriatr Oncol 4: 282-290.
-
Jiang H, Zhang X, Chen J, Zhang L, Xiong J, et al. (2014) A study of weekly docetaxel and carboplatin as first-line chemotherapy for advanced non-small cell lung cancer. J Thorac Dis 6: 79-85.
-
Shimizu T, Yokoi T, Tamaki T, Kibata K, Inagaki N, et al. (2013) Comparative analysis of carboplatin and paclitaxel combination chemotherapy schedules in previously untreated patients with advanced non-small cell lung cancer. Oncol Lett 5: 761-767.
-
Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, et al. (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378: 1079-1088.
-
Shitara K, Matsuo K, Oze I, Mizota A, Kondo C, et al. (2011) Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol 68: 301-307.
-
Giroux Leprieur E, Lavole A, Ruppert AM, Gounant V, Wislez M, et al. (2012) Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology 17: 134-142.
-
Drevs J, Fakler J, Eisele S, Medinger M, Bing G, et al. (2004) Antiangiogenic potency of various chemotherapeutic drugs for metronomic chemotherapy. Anticancer Res 24: 1759-1763.
-
De Iuliis F, Salerno G, Taglieri L, Lanza R, Scarpa S (2015) On and off metronomic oral vinorelbine in elderly women with advanced breast cancer. Tumori 101: 30-35.
-
Rajdev L, Negassa A, Dai Q, Goldberg G, Miller K, et al. (2011) Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol 68: 1119-1124.





