International Journal of Cancer and Clinical Research
Phase I Clinical Study of Survivin-Derived Peptide Vaccine for Patients with Advanced Gastrointestinal Cancers
Fukino Satomi1, Hiroaki Shima1, Toru Mizuguchi1*, Toshihiko Torigoe2*, Goro Kutomi1, Yasutoshi Kimura1, Yoshihiko Hirohashi2, Yasuaki Tamura2, Tomohide Tsukahara2, Takayuki Kanaseki2, Akari Takahashi2, Hiroko Asanuma2, Yoichi M. Ito3, Hiroshi Hayashi4, Osamu Sugita4, Noriyuki Sato2 and Koichi Hirata1
1Department of Surgery, Surgical Oncology and Science, Sapporo Medical University School of Medicine, Japan
2Department of Pathology, Sapporo Medical University School of Medicine, Japan
3Department of Biostatistics, Hokkaido University Graduate School of Medicine, Japan
4Division of TR Planning and Management, Center for Translational Research, Hokkaido University, Japan
*Corresponding author:
Toru Mizuguchi, MD, PhD, Department of Surgery, Surgical Oncology and Science, Sapporo Medical University School of Medicine, S-1, W-16, Cho-ku, Sapporo, Hokkaido 060-8556, Japan, Tel: +81-11-611-2111 (ext. 3281), Fax: +81-11-613-1678, e-mail: tmizu@sapmed.ac.jp
Toshihiko Torigoe, MD, PhD, Department of Pathology, Sapporo Medical University School of Medicine, S-1, W-17, Cho-ku, Sapporo, Hokkaido 060-8556, Japan, Tel: +81-11-611-2111 (ext. 2691), Fax: +81-11-643-2310, E-mail: torigoe@sapmed.ac.jp
Int J Cancer Clin Res, IJCCR-2-012, (Volume 2, Issue 1), Original article; ISSN: 2378-3419
Received: January 22, 2015 | Accepted: February 02, 2015 | Published: February 04, 2015
Citation: Satomi F, Shima H, Mizuguchi T, Torigoe T, Kutomi G, et al. (2015) Phase I Clinical Study of Survivin-Derived Peptide Vaccine for Patients with Advanced Gastrointestinal Cancers. Int J Cancer Clin Res 2:012. 10.23937/2378-3419/2/1/1012
Copyright: © 2015 Satomi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Survivin is a member of the Inhibitor of Apoptosis Protein (IAP) family. It is expressed in fetal tissues but not in normal adult tissues. Since Survivin is over expressed in various types of tumor tissues as well as tumor cell lines, it is considered to be suitable as a target antigen for cancer vaccine therapy. We identified an HLA-A24-restricted antigenic peptide, SVN-2B (AYACNTSTL), derived from a splicing variant of Survivin-2B. In the present study, we carried out a phase I clinical study assessing the safety and efficacy of vaccination with the peptide in patients having advanced gastrointestinal cancer. Vaccinations with 0.1mg, 1.0mg, or 3.0mg doses of the SVN-2B peptide were given subcutaneously four times at 14-day intervals. In 20 patients who received at least one vaccination, grade 1 and grade 2 treatment-related adverse events were observed, including injection site extravasation (grade 2), injection site reaction (grade 1), skin induration (grade 1) and fever (grade 1). No severe adverse event was observed in any patient. Based on tumor size evaluated by computed tomography, eight of the 15 patients who completed the vaccination schedule were considered to have stable disease as assessed by the RECIST criteria. Analysis of peripheral blood lymphocytes using HLA-A24/peptide tetramers revealed the highest increase of SVN-2B-specific cytotoxic T lymphocyte frequency in the 1.0mg dose group. The present clinical study indicates that SVN-2B peptide vaccination is safe and can be considered a potent immunotherapy for HLA-A24-positive gastrointestinal cancer patients.
Keywords
Survivin, Cancer vaccine, Gastrointestinal cancer, Tetramer, Phase I trial
Abbreviations
IAP: Inhibitor of Apoptosis Protein, CTLs: Cytotoxic T lymphocytes, HLA: Human Leukocyte Antigen, CT: Computed Tomography, PBLs: Peripheral Blood Lymphocytes, AEs: Adverse Events, HIV: Human Immunodeficiency Virus, PD: Progressive Disease, SD: Stable Disease, IFN: Interferon
Introduction
Cytotoxic T lymphocytes (CTLs) can recognize MHC class I-bound peptides derived from tumor antigens in cancer cells. Following the first report of the identification of a human tumor antigen, melanoma antigen-1 (MAGE-1), in 1991 [1] a large number of antigenic peptides from various human cancers have been identified [2-7]. They have been employed in immunotherapy for cancer and clinical trials of peptide-based vaccine therapies have taken place [8-11].
We have identified a human leukocyte antigen (HLA)-A24-restricted antigenic peptide, SVN-2B (AYACNTSTL), which was derived from the exon 2B-encoded region of Survivin-2B, a splicing variant of Survivin [12]. Survivin is a member of the inhibitor of apoptosis protein (IAP) family with a single baculovirus IAP repeat domain [13]. It is expressed during fetal development but undetectable in terminally differentiated normal adult tissues. In contrast to normal tissues, Survivin and Survivin-2B are expressed in transformed cell lines and in most common cancers, including gastrointestinal cancer and pancreatic cancer [13,14]. We reported previously that SVN-2B peptide-specific CTLs were increased by stimulating peripheral blood lymphocytes (PBLs) of cancer patients with the peptide in vitro [15]. The induced CTLs showed specific cytotoxicity against HLA-A24-positive cancer cells [15-[17]. We have carried out clinical trials of SVN-2B vaccination. The SVN-2B peptide was given subcutaneously to patients six times or more at biweekly intervals for colon, breast, oral cavity, and urinary bladder cancer patients [18-24]. There were no severe adverse effects and, clinically, certain patients showed reductions in tumor markers and tumor size as assessed by Computed Tomography (CT). In the present clinical study, we reevaluated the safety and efficacy of SVN-2B vaccination in accordance with good clinical practice guidelines and evaluated the optimal dose of the peptide.
Methods
Patient selection
The study protocol was approved by the Institutional Review Board of Sapporo Medical University. All patients gave informed consent before being enrolled. This study was conducted in accordance with the International Conference on Harmonisation E6 requirements for Good Clinical Practice and with the ethical principles outlined in the Declaration of Helsinki.
Patients enrolled in this study were required to conform to the following criteria: (1) to have histologically confirmed gastrointestinal, bile duct, or pancreatic cancer, (2) to be HLA-A*2402 positive, (3) to have Survivin-positive cancer tissue confirmed by immunohistochemical staining, (4) to be between 20 and 85 years old, (5) to have lesions measureable by CT at the time of registration, (6) to have a history of standard chemotherapy, (7) to have grade 0 or 1 in Eastern Cooperative Oncology Group (ECOG) performance status, and (8) to have no serious organ failure within 30 days at the time of registration.
Exclusion criteria included: (1) prior cancer therapy such as chemotherapy, radiation therapy or other immunotherapy within the previous 4 weeks, (2) presence of other cancers that might influence the prognosis, (3) administration of immunosuppressive drugs such as systemic steroid therapy, (4) severe cardiac insufficiency, acute infection, or hematopoietic failure, (5) uncontrollable diabetes or hypertension, (6) pregnancy or ongoing breast-feeding, and (7) unsuitability for the trial based on clinical judgment. In addition, patients with a high frequency of the peptide-specific CTLs at the time of registration were excluded since such patients were poor responders to the vaccine in our previous studies [23,24]. The number of the HLA-A24/SVN-2B peptide tetramer-positive CTLs per 10,000 CD8-positive T cells (CTLpre) was analyzed at the time of registration and patients who had a value of log10 (1+CTLpre) higher than 1.6 were excluded.
Peptide preparation
The peptide SVN-2B with the sequence AYACNTSTL was prepared under good manufacturing practice conditions by PolyPeptide Laboratories San Diego (San Diego, CA, USA). The identity of the peptide was confirmed by mass spectral analysis, and the purity was shown to be more than 98% as assessed by high pressure liquid chromatography analysis. The peptide was supplied as a freeze-dried, sterile white powder. It was dissolved in 1.0 ml of physiological saline (Ohtsuka Pharmaceutical Co., Ltd., Tokyo, Japan) and stored at -80°C until just before use.
Patient treatment
This study was carried out as an open-label, randomized parallel group study at the Department of Surgery, Surgical Oncology and Science of Sapporo Medical University Hospital to evaluate the safety and efficacy of the SVN-2B peptide vaccine for patients who had advanced or recurrent gastrointestinal or pancreatic cancer (UMIN000008611). The patients were randomly assigned into the following three dosage groups: group 1 patients received 0.1mg, group 2 received 1.0mg and group 3 received 3 mg. Each group included five patients. SVN-2B at a dose of 0.1mg/1mL, 1 mg/1mL, or 3 mg/1mL was emulsified with Montanide ISA51VG (Seppic, Paris, France) at a volume of 0.8 mL immediately before vaccination. The patients were then vaccinated subcutaneously (s.c.) four times at 14-day intervals (Figure 1).
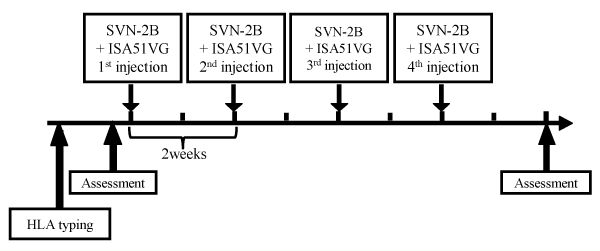
Figure 1: Protocols of the clinical study
The SVN-2B peptide at a dose of 0.1 mg/1mL, 1 mg/1 mL, or 3 mg/1 mL was emulsified with Montanide ISA51VG at a volume of 0.8 mL immediately before
vaccination. The patients were then vaccinated subcutaneously (s.c.) four times at 14-day intervals. Tumor size and the immunological response were
evaluated before treatment and at two weeks after the 4th vaccination.
View Figure 1
Toxicity evaluation
Patients were examined closely for signs of toxicity during and after vaccination. Adverse events (AEs) were recorded using CTCAE (version 4.03) criteria and graded for severity.
Clinical response evaluation
Physical and hematological examinations were conducted before and after each vaccination. Changes in tumor marker levels (CEA and CA19-9) were evaluated by comparison of the serum levels before the first vaccination and those after the fourth vaccination. Tumor size was evaluated by CT scans before treatment and at two weeks after the fourth vaccination (Figure 1). The antitumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST: version 1.1) guideline [25]. Briefly, a complete response (CR) was defined as complete disappearance of all measurable and evaluable disease. A partial response (PR) was defined as a >=30% decrease from baseline in the size of all measurable lesions (sum of maximal diameters). Progressive disease (PD) was defined as an increase in the sum of maximal diameters by at least 20% or the appearance of new lesions. Stable disease (SD) was defined as the absence of criteria matching those for CR, PR or PD. Patients who received fewer than four vaccinations were excluded from clinical response evaluations in this study.
In vitro stimulation of PBLs
PBLs were isolated by Ficoll-Conray density gradient centrifugation using Lymphoprep (AXIS-SHIELD, Oslo, Norway). They were then frozen and stored at -80 C. The frozen PBLs were thawed and incubated in the presence of 40 µg/mL SVN-2B in AIM-V medium (Life Technologies, Carlsbad, CA, USA) containing 10% human serum at room temperature. Next, interleukin-2 was added at a final concentration of 50 U/mL 1 hour, 2 days and 4 days after addition of the peptide. On day 7 of culture, the PBLs were analyzed by tetramer staining assay and ELISPOT assay.
Tetramer staining
FITC-labeled HLA-A*2402/human immunodeficiency virus (HIV)-derived peptide (RYLRDQQLL) and PE-labeled HLA-A*2402/SVN-2B peptide tetramers were purchased from MBL, Inc. (Nagoya, Japan). For flow cytometric analysis, PBLs, which were stimulated in vitro as above, were stained with the FITC-labeled tetramer and PE-labeled tetramer at 37°C for 20 min, followed by staining with a PC5-labeled anti-CD8 monoclonal antibody (Beckton Dickinson Biosciences, San Jose, CA, USA) at 4°C for 30 min. The cells were washed twice with PBS before fixation in 1% formaldehyde. Flow cytometric analysis was performed using FACSCalibur and CellQuest software (Beckton Dickinson Biosciences). The frequency of CTL precursors was calculated as the number of HLA-A24/SVN-2B tetramer-positive cells per 10,000 CD8-positive cells.
ELISPOT assay
ELISPOT plates were coated sterilely overnight with an IFN-γ capture antibody (Beckton Dickinson Biosciences) at 4°C. The plates were then washed once and blocked with AIM-V medium containing 10% human serum for 2 h at room temperature. CD8-positive T cells separated from patients' PBLs (5x103 cells/well), which were stimulated in vitro as above, were then added to each well along with HLA-A24-transfected CIR cells (CIR-A24) (5x104 cells/well) preincubated with SVN-2B (10 ng/mL, 100 ng/mL, 10 µg/mL) or the HIV peptide (RYLRDQQLL) as a negative control. After incubation in a 5% CO2 humidified chamber at 37°C for 24 h, the wells were washed vigorously five times with PBS and incubated with a biotinylated anti-human IFN-γ detection antibody (Beckton Dickinson Biosciences) and horseradish peroxidase-conjugated avidin. Spots were visualized and analyzed using KS ELISPOT (Carl Zeiss, Jena, Germany).
Immunohistochemistry
Immunohistochemical study of the HLA class I expression in the patients' primary cancer tissues was done with anti-HLA class I heavy chain monoclonal antibody EMR8-5 according to the standard methods described previously [26].
Statistical analysis
All statistical analyses were done using SAS Version 9.3 and JMP Version 11.0 (SAS Institute, Inc.). For the tetramer assay, statistical analysis was performed using a one-sided t-test. Statistical analysis of ELISPOT assay was performed using the student t-test.
Results
Patient profiles
From August 2012 to May 2013, 38 patients were assessed for eligibility and 21 patients were initially enrolled in this trial (Figure 2). However, one patient was withdrawn before the first vaccination due to deterioration of the systemic condition. Twenty patients who received at least one vaccination were evaluated for safety as a full analysis set (FAS). Five patients discontinued halfway through the protocol due to progression of the disease. None of the interruptions was due to treatment-related AEs. Fifteen patients received the complete regimen including four vaccinations and were evaluated for efficacy of the vaccine (Figure 2). The patient profiles are shown in Table 1. The primary malignant tumors of the 20 patients were 12 pancreatic cancers, 6 colon cancers (including 2 appendix cancers), 1 gastric cancer and 1 bile duct cancer.
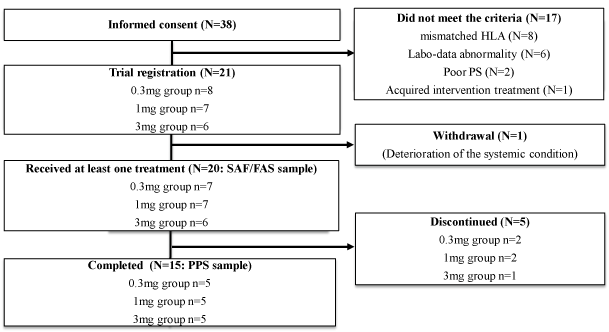
Figure 2: Enrollment of patients
Thirty-eight patients were assessed for eligibility and 21 were initially enrolled in this trial. One patient was withdrawn before the first vaccination due to
deterioration of the systemic condition. Twenty patients who received at least one vaccination were evaluated for safety as the full analysis set. Five
patients discontinued halfway through the protocol due to progression of the disease. Fifteen patients received the complete regimen and were evaluated
for efficacy of the vaccine as the per protocol set. SAF: Safety Analysis Set, FAS: Full Analysis Set, PPS: Per Protocol Set, HLA: Human Leukocyte Antigen,
PS: Performance Status.
View Figure 2
![]()
Table 1: Profiles of patients in the full analysis set for safety assessment (N=20)
View Table 1
Safety
Peptide vaccination was well tolerated in all patients. The treatment-related AEs are listed in Table 1. They included injection site extravasation (grade 2), injection site reaction (grade 1), skin induration (grade 1) and fever (grade 1). No serious toxicity-associated adverse event was observed during or after the vaccination.
Clinical responses
Table 2 summarizes the clinical outcomes of the 15 patients who received the complete regimen. CT evaluation of tumor size showed that 8 patients had SD and 7 patients PD, although none had PR or CR. The disease control rate was 53.3%. Among the 8 patients who were defined as having SD, the CEA levels and the CA19-9 levels were decreased or at least stable during vaccination in 2 patients and 3 patients, respectively. The CEA levels stayed within the normal range (0~5.9 ng/ml) throughout the study in 4 patients, and the CA19-9 level stayed within the normal range (0~37 U/ml) in one patient. It was noted that all three patients who had undergone immunotherapy before the registration had PD. Moreover, the result for one patient who had HLA class I-negative cancer tissue was also PD.
![]()
Table 2: Profiles and clinical outcomes of patients who completed the regimen
View Table 2
Tetramer assay and ELISPOT assay
We investigated whether the SVN-2B peptide vaccination could actually induce specific immune responses in the enrolled patients. The peptide-specific CTL frequencies in PBLs before the first vaccination (CTLpre) and after the fourth vaccination (CTLpost) were assessed using the HLA-A24/SVN-2B tetramer, and the tetramer increase (CTLpost-CTLpre) was calculated (Table 2). The HLA-A24/HIV peptide (RYLRDQQLL) tetramer was used as a negative control. SVN-2B-specific CTL frequencies were increased after the vaccination in all patients except two who had undergone immunotherapy before the registration. We compared the tetramer increases between the PD group (non-responders) and SD group (responders). The mean tetramer increase of the SD group was higher than that of the PD group (Figure 3A), although there was no statistical significance (p=0.19). To determine the optimal dose of the peptide to induce specific CTLs in patients, the mean tetramer increase indices of the three groups (0.1 mg dose, 1.0mg dose, and 3.0 mg dose) were compared (Figure 3C). It was found that 1.0mg was the most effective dose for the induction of peptide-specific T cells after the fourth vaccination (p=0.0046).
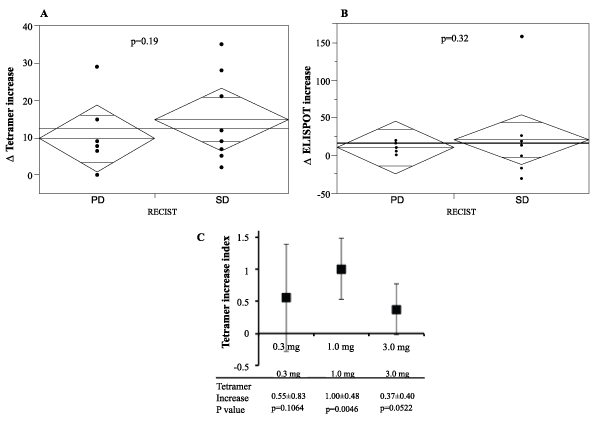
Figure 3: Tetramer assay and ELISPOT assay
(A) The tetramer increase (CTLpost-CTLpre) was calculated from the peptide-specific CTL frequency in PBLs before the first vaccination (CTLpre) and after
the fourth vaccination (CTLpost) using the HLA-A24/SVN-2B tetramer. The mean tetramer increases of the PD (non-responders) and SD groups (responders)
were compared. (B) The ELISPOT increase was calculated from the numbers of the peptide-specific IFN-γ spots before the first vaccination and after the fourth
vaccination. The mean ELISPOT increases of the PD and SD groups were compared. (C) The mean tetramer increase index was calculated according to the
following formula: Tetramer increase index=Log10(1+CTLpost)-Log10(1+CTLpre). The mean tetramer indices of the three groups (0.1 mg dose, 1.0 mg dose,
and 3.0 mg dose) were compared.
View Figure 3
We also analyzed the peptide-specific IFN-γ responses of CD8-positive T cells by ELISPOT assay. The HIV peptide (RYLRDQQLL) was used as a negative control. The numbers of peptide-specific IFN-γ spots before the first vaccination and after the fourth vaccination were counted, and the ELISPOT increase was calculated (Table 2). There was no significant difference in the mean ELISPOT increase between the SD group and PD group (Figure 3B).
Overall, this study suggests that the immunological response of the vaccine is well represented by tetramer assay rather than ELISPOT assay and that the immunological responses, at least in some patients, appropriately reflect the antitumor responses.
A Case Study
An 84-year-old woman who had primary colon cancer and metastatic liver and pancreatic tumors received the 1.0 mg dose of the SVN-2B vaccine. CT images and tetramer staining data are shown in Figure 4. In this case, the clinical response was defined as SD, and the peptide-specific CTL frequency was increased after the vaccination (Figure 4A). The metastatic pancreatic tumor barely changed from 16 mm to 17 mm during the 8 weeks of the study (Figure 4B). She continued the vaccination after the study. After 6 months, the pancreatic tumor size had increased by 31%, and a new lesion appeared in the caudate lobe of the liver. The time to progression was 267 days. There was no treatment-related AE and she could maintain high quality of daily life for almost one and a half years.
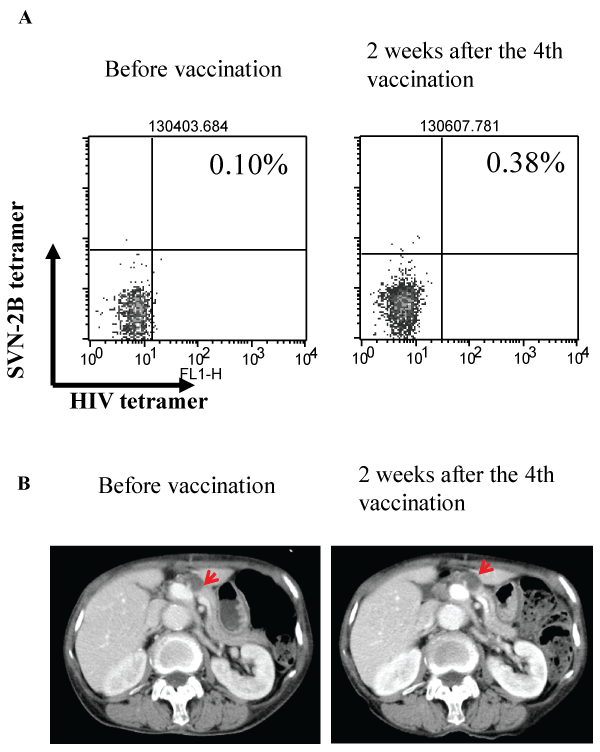
Figure 4: Tetramer assay and CT scan images of the patient with a metastatic pancreatic tumor
An 84-year-old woman with primary colon cancer and metastatic pancreatic tumor. (A) Tetramer assay before vaccination (left panel) and 2 weeks after the
4th vaccination (right panel). (B) CT scan images before vaccination (left panel) and 2 weeks after the 4th vaccination (right panel). The arrowhead indicates
the metastatic pancreatic tumor. The tumor grew slightly from 16 mm to 17 mm during the 8 weeks of the study.
View Figure 4
Discussion
The present study demonstrated the safety and clinical efficacy of the survivin-2B peptide vaccine for patients with advanced gastrointestinal cancer. However, the efficacy of vaccination with the SVN-2B peptide plus oil adjuvant Montanide ISA51VG was limited and not sufficient to elicit overt clinical responses. It is obvious that superior clinical and immunological responses are necessary for cancer immunotherapy. It should be considered that vaccination in combination with immunostimulatory adjuvants or cytokines may lead to greater immune and clinical responses. We have reported that type I interferon (IFN) can enhance the antitumor and immunological responses of the peptide vaccine [19,20]. On the basis of the results in this phase I study, we have started a phase II study of the SVN-2B peptide vaccine in combination with IFN-γ.
Immunomonitoring revealed that the tetramer increases were well correlated with antitumor responses as compared with ELISPOT analysis. Therefore, we used the tetramer increase as an index of vaccine-specific immune responses and determined the optimal peptide dose. A significantly higher frequency of tetramer-positive CTLs was induced in the 1mg dose group. However, the optimal dose may vary depending on conditions such as the vaccination interval and combination with distinct adjuvants and/or cytokines, and may have to be reevaluated in combination with IFN. It is enigmatic why the 3 mg dose vaccination caused less induction of the peptide-specific CTLs. It was reported previously that persistent vaccine depots could induce sequestration, dysfunction and depletion of antigen-specific CTLs [27]. That may explain, at least in part, the mechanism of the bell-shaped dose effect of the antigenic peptide.
Three patients with a history of immunotherapy such as a dendritic cell vaccine and certain peptide vaccine failed to respond to the SVN-2B peptide vaccine clinically and immunologically. It is possible that their cancers may have had immunoescape phenotypes, thereby maintaining resistance to the vaccine as well as the prior immunotherapy. Alternatively, prior immunotherapy might have affected the immune system, thereby inducing tolerance against the vaccination. In any case, a history of immunotherapy was considered to be a predictive factor for a worse response, and was therefore added to the exclusion criteria in the ongoing phase II clinical study.
In conclusion, we demonstrated the safety and clinical efficacy of the SVN-2B peptide vaccine for patients with advanced gastrointestinal cancer, although clinical interpretation of the results was limited due to this being a phase I study with a small number of patients. At present, a phase II study (UMIN000012146) of the SVN-2B peptide vaccine for advanced pancreatic cancer is ongoing in combination with IFN-γ.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant Nos. 16209013, 17016061 and 15659097) for Practical Application Research from the Japan Science and Technology Agency, and for Cancer Research (15-17 and 19-14) from the Ministry of Health, Labor and Welfare of Japan, Ono Cancer Research Fund and Takeda Science Foundation. This work was supported in part by the National Cancer Center Research and Development Fund (23-A-44) and the Grant for Joint Research Project of the Institute of Medical Science, the University of Tokyo.
References
-
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, et al. (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254: 1643-1647.
-
Nakatsugawa M, Horie K, Yoshikawa T, Shimomura M, Kikuchi Y, et al. (2011) Identification of an HLA-A*0201-restricted cytotoxic T lymphocyte epitope from the lung carcinoma antigen, Lengsin. Int J Oncol 39: 1041-1049.
-
Honma I, Torigoe T, Hirohashi Y, Kitamura H, Sato E, et al. (2009) Aberrant expression and potency as a cancer immunotherapy target of alpha-methylacyl-coenzyme A racemase in prostate cancer. J Transl Med 7: 103.
-
Kawaguchi S, Tsukahara T, Ida K, Kimura S, Murase M, et al. (2012) SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci 103: 1625-1630.
-
Sato E, Torigoe T, Hirohashi Y, Kitamura H, Tanaka T, et al. (2008) Identification of an immunogenic CTL epitope of HIFPH3 for immunotherapy of renal cell carcinoma. Clin Cancer Res 14: 6916-6923.
-
Tsukahara T, Kawaguchi S, Torigoe T, Takahashi A, Murase M, et al. (2009) HLA-A*0201-restricted CTL epitope of a novel osteosarcoma antigen, papillomavirus binding factor. J Transl Med 7: 44.
-
Hariu H, Hirohashi Y, Torigoe T, Asanuma H, Hariu M, et al. (2005) Aberrant expression and potency as a cancer immunotherapy target of inhibitor of apoptosis protein family, Livin/ML-IAP in lung cancer. Clin Cancer Res 11: 1000-1009.
-
Rosenberg SA (1999) A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 10: 281-287.
-
Tsuruma T, Hata F, Furuhata T, Ohmura T, Katsuramaki T, et al. (2005) Peptide-based vaccination for colorectal cancer. Expert Opin Biol Ther 5: 799-807.
-
Kawaguchi S, Wada T, Ida K, Sato Y, Nagoya S, et al. (2005) Phase I vaccination trial of SYT-SSX junction peptide in patients with disseminated synovial sarcoma. J Transl Med 3: 1.
-
Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H (2013) Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med 11: 291.
-
Hirohashi Y, Torigoe T, Maeda A, Nabeta Y, Kamiguchi K, et al. (2002) An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res 8: 1731-1739.
-
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3: 917-921.
-
Asanuma H, Torigoe T, Kamiguchi K, Hirohashi Y, Ohmura T, et al. (2005) Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res 65: 11018-11025.
-
Idenoue S, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, et al. (2005) A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 11: 1474-1482.
-
Kitamura H, Torigoe T, Honma I, Asanuma H, Nakazawa E, et al. (2006) Expression and antigenicity of survivin, an inhibitor of apoptosis family member, in bladder cancer: implications for specific immunotherapy. Urology 67: 955-959.
-
Kobayashi J, Torigoe T, Hirohashi Y, Idenoue S, Miyazaki A, et al. (2009) Comparative study on the immunogenicity between an HLA-A24-restricted cytotoxic T-cell epitope derived from survivin and that from its splice variant survivin-2B in oral cancer patients. J Transl Med 7: 1.
-
Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, et al. (2009) Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother 58: 1801-1807.
-
Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, et al. (2013) Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci 104: 124-129.
-
Kameshima H, Tsuruma T, Torigoe T, Takahashi A, Hirohashi Y, et al. (2011) Immunogenic enhancement and clinical effect by type-I interferon of anti-apoptotic protein, survivin-derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci 102: 1181-1187.
-
Miyazaki A, Kobayashi J, Torigoe T, Hirohashi Y, Yamamoto T, et al. (2011) Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci 102: 324-329.
-
Tanaka T, Kitamura H, Inoue R, Nishida S, Takahashi-Takaya A, et al. (2013) Potential survival benefit of anti-apoptosis protein: survivin-derived peptide vaccine with and without interferon alpha therapy for patients with advanced or recurrent urothelial cancer--results from phase I clinical trials. Clin Dev Immunol 2013: 262967.
-
Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, et al. (2004) Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2: 19.
-
Tsuruma T, Iwayama Y, Ohmura T, Katsuramaki T, Hata F, et al. (2008) Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med 6: 24.
-
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247.
-
Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, et al. (2012) Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int 62: 303-308.
-
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, et al. (2013) Persistent antigen at vaccination sites induces tumor-specific CD8⺠T cell sequestration, dysfunction and deletion. Nat Med 19: 465-472.





