International Journal of Brain Disorders and Treatment
Decreased Hippocampal Volume is Related to White Matter Abnormalities in Treatment-Resistant Depression
Christina B Young1,2,3*, Philip van Eijndhoven2,3, Robin Nusslock1, Guillén Fernández3,4, Aart Schene2,5, Christian F Beckmann3,4 and Indira Tendolkar2,3
1Department of Psychology, Northwestern University, Evanston IL, USA
2Department of Psychiatry, Radboud University Medical Center, Nijmegen, The Netherlands
3Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, The Netherlands
4Department of Cognitive Neuroscience, Radboud University Medical Center, Nijmegen, The Netherlands
5Department of Psychiatry, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
*Corresponding author:
Christina B Young, Department of Psychology, Northwestern University, Evanston IL, USA, E-mail: cbyoung@u.northwestern.edu
Int J Brain Disord Treat, IJBDT-2-012, (Volume 2, Issue 1), Original Article; ISSN: 2469-5866
Received: April 27, 2016 | Accepted: June 20, 2016 | Published: June 22, 2016
Citation: Young CB, Eijndhoven PV, Nusslock R, Fernández G, Schene A, et al. (2016) Decreased Hippocampal Volume is Related to White Matter Abnormalities in Treatment-Resistant Depression. Int J Brain Disord Treat 2:012. 10.23937/2469-5866/1510012
Copyright: © 2016 Young CB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Despite considerable research on the pathophysiology of unipolar depression, relationships between gray and white matter brain changes associated with treatment-resistant depression (TRD) have been sparsely investigated. Here, we used voxel-based morphometry and tract-based spatial statistics to examine differences in fronto-limbic gray matter volume and their connecting white matter tracts, respectively. We compared a well-characterized group of medication-free TRD patients (n = 22), who had homogenous treatment backgrounds consisting of standardized psychotherapeutic treatment and a combination of antidepressants and mood stabilizers, to matched healthy controls (n = 21). In comparison to healthy controls, TRD patients showed reduced gray matter volume in the left medial orbitofrontal cortex and bilateral hippocampus. Increased fractional anisotropy (FA) in the left angular bundle, and increased FA and mode of anisotropy (MO) in the right uncinate fasciculus were also found amongst TRD patients. Critically, left hippocampus volume was negatively correlated with FA values in the nearby left angular bundle in the TRD group only. Therefore, TRD was associated with concurrent reduced gray matter volume and aberrant white matter structure, and with, abnormalities in the left hippocampus and its related projections in particular. Development of treatments targeting this region may be fruitful for depression in general, and for this group of patients who are especially difficult to treat.
Keywords
Treatment-resistant depression, Voxel-based morphometry, Diffusion tensor imaging, Hippocampus
Introduction
Major depressive disorder (MDD) places an enormous burden on affected individuals, their caregivers and society as a whole [1,2]. Although new pharmacological and psychotherapeutic treatments for depression have been developed in the last decades, up to one third of depressed patients still go on to develop treatment-resistant depression (TRD) [3]. Identifying brain abnormalities associated with TRD may be informative about underlying mechanisms of the disorder and deliverable drug targets, and this is especially important because innovating treatment for MDD and TRD in particular is urgent.
The majority of TRD research studies use the European Staging Model, which operationalizes TRD as non-response to at least two different classes of antidepressants [4]. However, there is significant variation in the severity of patients classified as having TRD because this is a broad definition, and because other staging models defining TRD are also used [5]. Additionally, the majority of extant TRD studies typically examine patients currently on medication, and are therefore unable to disentangle current medication use from neural markers associated with TRD. To circumvent these limitations, we examined a homogenous sample of unmedicated TRD patients.
Following prominent network models of depression [6-8], we focused on fronto-limbic structures that have shown structural and functional abnormalities in multiple previous studies. In particular, we targeted the amygdala because it's volume has been shown to be impacted in MDD [9], the hippocampus because it is thought to be related to chronicity in MDD [10], and the anterior cingulate cortex (ACC) because it is often targeted in deep brain stimulation, a treatment for TRD [11]. Impaired micro structural integrity in white matter structures connecting these temporal and frontal regions, such as the cingulum bundle and uncinate fasciculus, have also been identified in TRD [12-15] and MDD [16-18]. Focusing on these fronto-limbic regions, we simultaneously examined gray and white matter structures in TRD using voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS), respectively.
VBM is a widely used technique that characterizes localized differences in gray matter volume between groups of subjects at the voxel level across the whole brain or within a-priori regions of interest. To examine white matter differences in quantities derived from diffusion weighted imaging data, we used a recently developed method called TBSS. Different measures based on the anisotropic diffusion of water in white matter tracts can be derived from this data under the diffusion tensor imaging (DTI) model. The most common is fractional anisotropy (FA), which quantifies the degree to which the voxel-wise estimated diffusivity proceeds along a single preferred direction, thereby providing information about microstructural tissue integrity. Mean diffusivity (MD), which measures total strength of diffusion to provide information about fiber density, as well as mode of anisotropy (MO), which reflects the diffusion shape to provide information about crossing fibers, can also be assessed.
The combined VBM and TBSS analyses are used here provide a clearer understanding of structural brain abnormalities associated with TRD by simultaneously identifying patterns of related change in gray and white matter. These techniques can be used to identify changes in volume and associated connectivity in TRD, and previous studies have demonstrated that both white and gray matter changes can be indicative of disease status [19-22]. Based on the extant literature, we hypothesized that individuals with TRD will show decreased gray matter volume and impaired white matter integrity in fronto-limbic regions in comparison to healthy controls. Furthermore, because previous studies have not related gray and white matter abnormalities to each other, we explored the relationship between imaging modalities.
Methods
Participants
Patients were recruited from the Department of Psychiatry of Radboud University Medical Centre Nijmegen, The Netherlands. All patients had undergone a stepwise treatment consisting of a combination of serotonin-reuptake inhibitors (SSRIs), serotonin-noradrenaline-reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase (MAO) inhibitors and mood stabilizers without clinical response, and were awaiting electroconvulsive therapy at the time of the scan. A diagnosis of MDD was established using the Structured Clinical Interview for DSM-IV (SCID-IV). Depressive symptoms were rated using the 17-item Hamilton Depression Rating Scale (HDRS, [23]) within one week of the scan. Although HDRS scores indicate that patients ranged from mild to severe depression, all patients had symptoms that interfered with daily functioning and the symptoms were not remitting.
To minimize the confounding effects of medication, all patients underwent a wash-out phase with a minimum duration of 1 week prior to the scan except for tranylcypromine, which was reduced over several weeks and stopped 3 weeks prior to the scan due to its irreversible binding qualities. None of the patients received an SSRI with a long-lasting half-life prior to the scan. Exclusion criteria were electroconvulsive therapy treatment within 1 year prior to the scan, bipolar depression, and a co-morbid diagnosis of schizophrenia or substance dependence disorder. Further exclusion criteria were current use of any psychotropic medication other than intermittent use of benzodiazepines, current or past relevant somatic or neurological disorders, and magnetic resonance imaging (MRI)-related exclusion criteria such as claustrophobia, pregnancy or a pacemaker.
Exclusion criteria for the healthy comparison subjects were any lifetime DSM-IV axis I disorder, as assessed with the Mini-International Neuropsychiatric Interview [24], and/or a history of psychiatric disorders in first-degree relatives. All participants were otherwise healthy and did not use any medication other than hormonal contraceptives. Other exclusion criteria were a history of substance abuse or dependence, a history of traumatic brain injury, claustrophobia, metal implants, and for women, postpartum depression, pregnancy, lactation, or menopause.
After excluding one healthy control due to poor DTI data, our final sample consisted of 22 TRD patients and 21 healthy controls. The groups were equivalent in gender distribution, Χ2(1, N = 43) = 0.014, p > 0.57, age, t(41) = -0.253, p > 0.80, handedness, Χ2(1, N = 43) = 0.282, p > 0.59, and education level, t(41) = 0.563, p > 0.57. Table 1 describes the TRD patient demographics and treatment history. The study was approved by the local medical ethical committee, commissie mensgebonden onderzoek Regio Arnhem-Nijmegen, and all subjects provided written informed consent.
![]()
Table 1: Treatment-resistant depression (TRD) patient characteristics, and summary of pharmacological treatment history.
View Table 1
Image acquisition
Structural MRI data were collected on a 1.5-Tesla Siemens Avanto scanner (Siemens, Erlangen, Germany). A T1-MPR-NS sequence was used with the following parameters: repetition time (TR) = 2.73 s, echo time (TE) = 2.95 ms, inversion time (TI) = 1000 ms, flip angle = 7 degrees, field of view = 256 × 256 × 256, and voxel-size = 1 × 1 × 1 mm. Diffusion weighted imaging data were collected using the following parameters: TR = 7.40 s, TE = 85 ms, field of view = 220, slice thickness = 2.5 mm, 56 interleaved slices. The diffusion sensitizing gradients were applied along 34 collinear directions (b = 1000 s/mm2). All data were processed using tools from the FMRIB Software Library (FSL, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/).
Gray matter VBM analysis
Structural data were analyzed using FSL-VBM [25], (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol [26] executed with FSL tools [27]. Structural images were brain-extracted, gray matter segmented, and registered to the MNI-152 standard space using non-linear registration. A study-specific gray matter template was created by averaging structural images from 21(randomly selected) TRD and 21 healthy controls. Next, all native gray matter images were non-linearly registered to this study-specific template and modulated to correct for local expansion or contraction due to the non-linear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm. The study-specific template and structural images for each participant were checked to ensure data quality.
To investigate differences in gray matter volume between TRD and healthy controls within our a-priori fronto-limbic regions of interest (ROIs), we used a permutation-based non-parametric inference method within the general linear model framework. The Harvard-Oxford probabilistic cortical atlas thresholded at 20% was used to identify the subcallosal cortex, which included medial orbitofrontal cortex (OFC), and the Harvard-Oxford probabilistic subcortical atlas thresholded at 20% was used to identify the bilateral amygdala and hippocampus. Group differences were examined within this mask (small-volume corrected) and results were thresholded at p < .05 controlling the local false discovery rate [28].
White matter TBSS analysis
Voxelwise statistical analyses of diffusion weighted imaging data using FA, MD and MO indices were carried out using TBSS [27,29]. FA provides information about micro structural tissue integrity through diffusivity measurements, MD serves as a proxy for fiber density through assessment of total diffusion strength [30], and MO provides information about crossing fibers through assessment of diffusion shape [31]. Using FSL, FA images were first created by fitting a tensor model to the raw diffusion data using FDT, and then brain-extracted using BET. FA data from all subjects were then aligned into a common space using the nonlinear registration tool FNIRT, which uses b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all tracts common to the group. Each subject's aligned FA, MD and MO data were then projected onto this skeleton and the resulting data were fed into voxelwise cross-subject statistics. To define white matter ROIs, a fronto-limbic mask containing bilateral cingulum (hippocampus), cingulum (cingulate gyrus) and uncinate fasciculus was created using the JHU white-matter tractography atlas thresholded at 20%. Although these masks primarily contained the cingulum and uncinate fasciculus, they also contained smaller bundles, such as the angular bundle. Group differences within this white-matter mask (small-volume corrected) were examined and thresholded at p < 0.05 controlling the local false discovery rate [28]. Only clusters greater than or equal to 3 voxels are reported.
Results
Fronto-limbic gray matter differences
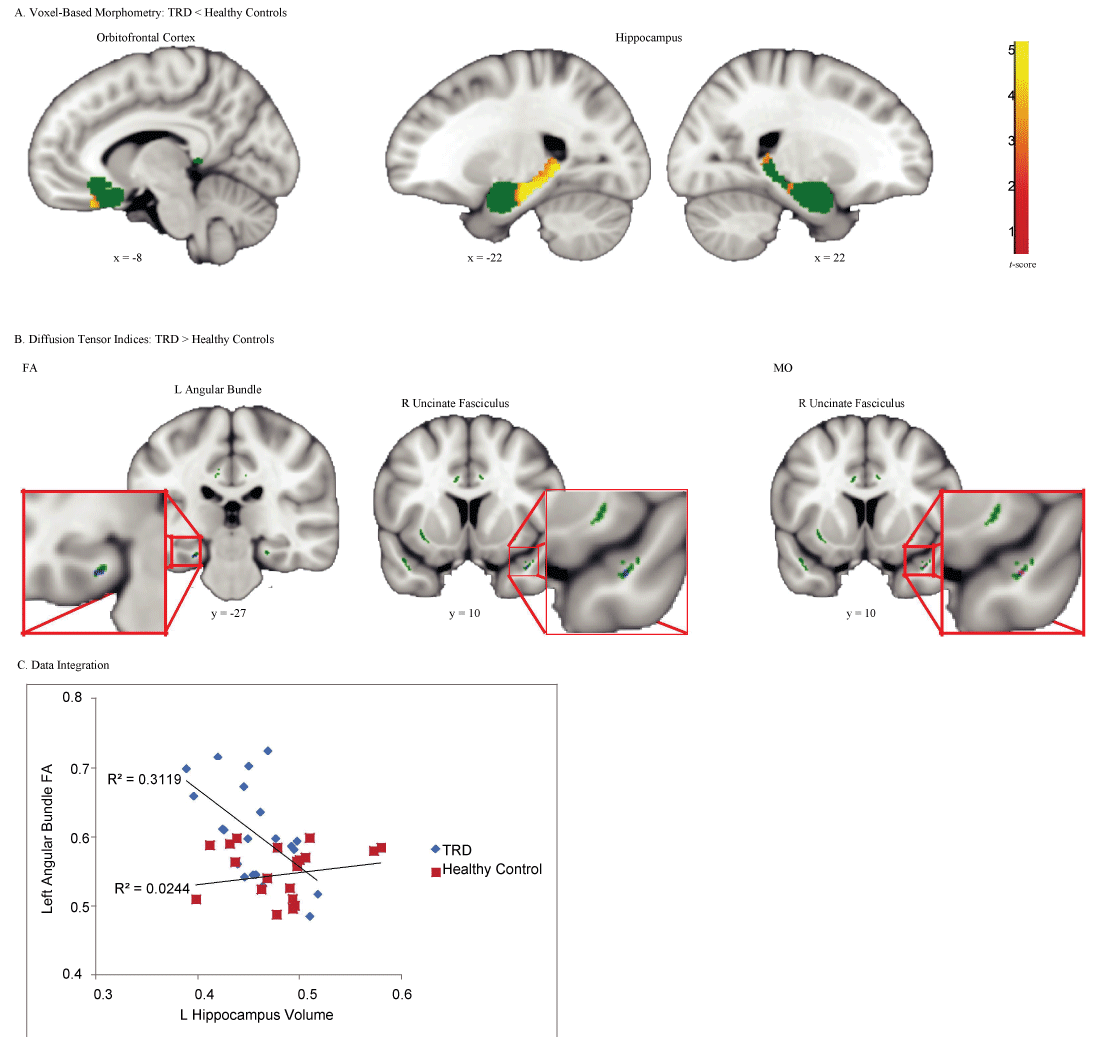
TRD patients showed reduced gray matter volumes relative to healthy controls in the left medial OFC and bilateral hippocampus, with a particularly prominent cluster in the left hippocampus (Figure 1A and Table 2). No regions showed greater gray matter volume in TRD patients than healthy controls.
.
Figure 1: Regions that showed significant differences between treatment-resistant depression (TRD) and healthy controls.
(A) Voxel-based morphometry revealed reduced gray matter volume in the TRD patients in comparison to healthy controls in the left medial orbitofrontal cortex and bilateral hippocampus. Green regions depict gray matter ROI mask consisting of the subcallosal cortex, amygdala, and hippocampus. (B) Diffusion tensor imaging showed increased fractional anisotropy (FA) in the left angular bundle and right uncinate fasciculus, as well as increased mode of anisotropy (MO) in the right uncinate fasciculus in TRD patients in comparison to healthy controls. Green regions depict white matter ROI mask consisting of bilateral cingulum (hippocampus), cingulum (cingulate gyrus), and uncinate fasciculus. (C) FA values in the left angular bundle were negatively correlated with gray matter volume in the left hippocampus in the TRD group only.
View Figure 1
Fronto-limbic white matter differences
Elevated FA values in the left angular bundle and right uncinate fasciculus, as well as elevated MO values in the overlapping right uncinate fasciculus were found in TRD patients in comparison to healthy controls (Figure 1B and Table 2). No regions showed decreased FA or MO in TRD patients versus healthy controls, and no differences in MD in either direction were found.
![]()
Table 2: Regions that showed significant structural differences between treatment-resistant depression (TRD) and healthy control groups.
View Table 2
Data integration
Due to the proximity of the significant left hippocampus volume cluster and the left angular bundle FA cluster, we next examined whether these values were correlated within the TRD group. Volume values in the left hippocampus cluster that showed a significant difference between groups identified in the VBM analysis, as well as FA values from the left angular bundle cluster identified in the TBSS analysis were extracted. One patient had a left hippocampus volume that was more than 2.5 standard deviations smaller than the TRD group mean. After excluding this outlier patient, the correlation between left hippocampus volume and left angular bundle FA in the TRD group was highly significant (r = -0.558, p = 0.009) (Figure 1C). Left hippocampus volume and left angular bundle FA were not significantly correlated in healthy controls (r = 0.156, p = 0.499).
Furthermore, we examined the relationship between hippocampal volume and DTI measures in the entire uncinate fasciculus and cingulum, as defined by a-priori ROIs, for the left and right hemispheres separately. After excluding outliers who had values greater than 2.5 standard deviations away from the TRD group mean, hippocampal volume in the region defined a-priori was not associated with FA, MD or MO in any of the white matter ROIs in TRD patients, all ps > 0.07. This suggests that there is a unique and specific relationship between the left hippocampus and left angular bundle in TRD patients, rather than a more global association between temporal lobe regions.
Discussion
Here, we simultaneously used VBM and TBSS methods to demonstrate that TRD is associated with patterns of related change in gray and white matter structures. By examining a unique homogeneous group of medication-free TRD patients who failed to respond to standardized psychotherapeutic treatment as well as a combination of antidepressants and mood stabilizers, we showed that compared to controls, TRD was associated with reduced gray matter volume in bilateral hippocampus and left medial OFC, with particularly large effects in the left hippocampus. Increased FA in the nearby left angular bundle was also found in the TRD group in comparison to healthy controls. Diffusion results were integrated with volumetric findings such that FA values in the left angular bundle were negatively correlated with gray matter volume in the left hippocampus in the TRD group only. This suggests that TRD may be characterized by abnormalities in the left hippocampus and its related fiber tracts.
The hippocampus is not only featured in fronto-limbic circuit models of depression [6-8], but is also thought to be central to the etiology and treatment of the disorder [32,33]. Smaller hippocampal volume has been shown to be a vulnerability factor for poor treatment response [34,35], and electroconvulsive therapy, a treatment that leads to remission in about 50% of depressed patients [36], has been shown to specifically increase hippocampal and amygdala volume [37]. Functionally, cells within the dentate gyrus structure of the hippocampus are thought to be especially relevant to depression as depression-like behavior was shown to be suppressed when specific cells in this region were stimulated in mice [38]. Our results showing reduced hippocampal volume in TRD patients may reflect a lack of neurogenesis in this region, which may then contribute to poor treatment response in this group.
Combining VBM results with tract-based white matter analyses allowed us to relate changes in gray matter to associated white matter abnormalities. Results from this study revealed that TRD was associated with increased FA in the left angular bundle adjacent to the left hippocampus region that showed reduced gray matter volume. The angular bundle is the primary route by which neocortical inputs reach the dentate gyrus and hippocampus, and is the main pathway for fibers that originate from the entorhinal cortex and project to other hippocampal components [39]. The increase in FA may thus reflect reduced integrity in smaller crossing fibers that are perpendicular to the dominant angular bundle, or reduced branching of white matter tracts [40]. Notably, the FA values in the left angular bundle were significantly negatively correlated with the volume of the nearby left hippocampus, suggesting that gray matter deficits in the hippocampus are associated with white matter abnormalities in hippocampus-related pathways. Thus, TRD is associated with abnormalities in numerous related mediotemporal structures. The significance of these left-lateralized results remains to be explored, especially as the sample was predominantly right-handed.
We also identified volumetric reductions within the medial OFC, a region strongly connected to the hippocampus that is critical for reward [41] and emotion processing [42]. Our results are consistent with several meta-analyses showing volumetric reductions within frontal regions, especially in the ACC and OFC, in depression [43,44]. Two studies in particular have shown prefrontal abnormalities in more severe depression groups; reduced gray matter volume in right prefrontal lobe has been found in TRD [45], and smaller gray matter volume in bilateral OFC has been found in suicidal MDD [46]. Our results extend these findings and show that TRD is characterized by reduced gray matter volume in the left medial OFC. Further exploration is needed to determine whether the lateralization of this finding is due to a lack of power or is instead related to the functional lateralization of the prefrontal cortex [47]. Because the medial OFC is functionally connected with emotion- and reward-related regions including the amygdala, hippocampus, striatum and insula [41], structural abnormalities of the medial OFC may have far-reaching consequences, and be involved in the emotion and reward processing aberrations observed in TRD.
Abnormalities within the uncinate fasciculus, a white matter tract that connects the anterior temporal lobe with medial and lateral OFC [48], were also identified. More specifically, TRD patients showed increased FA and MO, reflecting greater directionality and fewer crossing fibers respectively, in the right uncinate fasciculus in comparison to healthy controls. Similar results of increased FA accompanied by reduced radial diffusivity, which reflects fewer obliquely oriented fibers, and increased longitudinal diffusivity, which reflects more longitudinally aligned fibers, have been found in the same region in bipolar disorder [49]. A critical study examining FA and MO as well as probabilistic tractography demonstrated that increased MO and co-localised FA, which is also observed in this study, was explained by relative preservation of one fiber track along with degeneration of a crossing fiber track [31]. Since there are other tracts crossing at this uncinate fasciculus region [48], such as the fornix, it is possible that increased FA in TRD patients may be due to preservation of particular white matter tracks accompanied by degeneration of other white matter tracks projecting to or from the hippocampus. Future studies using tractography are needed to confirm this hypothesis. Nevertheless, these results suggest that TRD may be related to selective deficits in the uncinate fasciculus, a core limbic system white matter tract involved in emotion processing [50].
It is important to note that the FA results reported in this study are opposite to the majority of the extant depression literature that has reported decreased FA values associated with depression [51], although these decreases were observed in a variety of regions [12-14]. One possible explanation for this discrepancy is that there may be important genetic polymorphisms moderating microstructural integrity reflected in FA. Indeed, brain-derived neurotrophic factor alleles have been shown to differentially affect FA values in left cingulum (rostral) in MDD [18]. Additionally, it is possible that FA values are reduced across a broader depression spectrum, but are increased within our especially homogeneous TRD sample (i.e., restriction of range).
Limitations
Several limitations of this study are important to consider. First, because we did not have a non-TRD depressed group, direct comparisons between TRD and non-TRD depression were not possible. As a result, the structural abnormalities in fronto-limbic regions identified in this study may not be specific to TRD and may instead be characteristic of depression more broadly. Second, although the patients in this study were free from current medication, it is not possible to determine if the abnormalities identified were due to past medication use. However, TRD by definition requires patients to have an extensive medication history so disentangling TRD from past medication use may be extremely difficult. Third, focused ROIs were used to examine gray and white matter abnormalities and future studies examining other brain systems will be important. Finally, the reported structural abnormalities were small and need to be replicated in order to determine their robustness. Relatedly, the use of more optimal scan parameters, particularly for diffusion weighted imaging, that have been developed since the time of data acquisition will provide better resolution to confirm the abnormalities identified here.
Conclusion
In summary, we combined VBM and TBSS to simultaneously identify structural abnormalities in gray and white matter in a particularly severe group of medication-free TRD patients. Identification of abnormal volume and white matter integrity within various regions of the fronto-limbic circuit provide more evidence for conceptualizing TRD and depression more broadly as an illness involving emotion-related brain circuits. Furthermore, the relationship between left hippocampal volume and angular bundle FA suggests that TRD is not only associated with isolated abnormalities in gray and white matter brain regions, but that these structural changes may occur together. As a result, development of treatments targeting mediotemporal structures may be fruitful for depressed individuals, and this group of patients who are especially difficult to treat.
Acknowledgements
None.
Ethical Statement
The study was approved by the local medical ethical committee, commissie mensgebonden onderzoek (CMO) Regio Arnhem-Nijmegen, and all subjects provided written informed consent.
References
-
Smith K (2014) Mental health: a world of depression. Nature 515: 181.
-
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015) The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76: 155-162.
-
Petersen T, Gordon JA, Kant A, Fava M, Rosenbaum JF, et al. (2001) Treatment resistant depression and axis I co-morbidity. Psychol Med 31: 1223-1229.
-
Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, et al. (1999) Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol 9:83-91.
-
Ruhé HG, van Rooijen G, Spijker J, Peeters FP, Schene AH (2012) Staging methods for treatment resistant depression. A systematic review. J Affect Disord 137: 35-45.
-
Mayberg HS (2009) Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 119: 717-725.
-
Elliott R, Zahn R, Deakin JF, Anderson IM (2011) Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 36: 153-182.
-
Drevets WC (2007) Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci 1121: 499-527.
-
Hamilton JP, Siemer, M, Gotlib IH (2008) Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry 13: 993-1000.
-
MacQueen G, Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16: 252-264.
-
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, et al. (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45: 651-660.
-
Guo WB, Liu F, Chen JD, Xu XJ, Wu RR, et al. (2012) Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry 38: 201-206.
-
de Diego-Adelino J, Pires P, Gomez-Anson B, Serra-Blasco M, Vives-Gilabert Y, et al. (2014) Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med 44: 1171-1182.
-
Zhou Y, Qin L-d, Chen J, Qian L-j, Tao J, et al. (2011) Brain microstructural abnormalities revealed by diffusion tensor images in patients with treatment-resistant depression compared with major depressive disorder before treatment. Eur J Radiol 80: 450-454.
-
Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, et al. (2014) Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry 4: e380.
-
Zhang A, Leow A, Ajilore O, Lamar M, Yang S, et al. (2012) Quantitative tract-specific measures of uncinate and cingulum in major 2depression using diffusion tensor imaging. Neuropsychopharmacology 37: 959-967.
-
de Kwaasteniet B, Ruhe E, Caan M, Rive M, Olabarriaga S, et al. (2013) Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry 74: 40-47.
-
Carballedo A, Amico F, Ugwu I, Fagan AJ, Fahey C, et al. (2012) Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am J Med Genet B Neuropsychiatr Genet 159: 537-548.
-
Hayakawa YK, Sasaki H, Takao H, Mori H, Hayashi N, et al. (2013) Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. J Affect Disord 144: 263-268.
-
Hayakawa YK, Sasaki H, Takao H, Hayashi N, Kunimatsu A, et al. (2014) Depressive symptoms and neuroanatomical structures in community-dwelling women: A combined voxel-based morphometry and diffusion tensor imaging study with tract-based spatial statistics. NeuroImage Clin 4: 481-487.
-
Sprengelmeyer R, Orth M, Muller HP, Wolf RC, Gron G, et al. (2014) The neuroanatomy of subthreshold depressive symptoms in Huntington's disease: a combined diffusion tensor imaging (DTI) and voxel-based morphometry (VBM) study. Psychol Med 44: 1867-1878.
-
Han KM, Choi S, Jung J, Na KS, Yoon HK, et al. (2014) Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord 155: 42-48.
-
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56-62.
-
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22-33.
-
Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, et al. (2007) Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130: 2375-2386.
-
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, et al. (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21-36.
-
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1: S208-219.
-
Efron B (2004) Large-scale simultaneous hypothesis testing: the choice of a null hypothesis. J Amer Statistical Assoc 99: 96-104.
-
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, et al. (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487-1505.
-
Basser PJ (1995) Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333-344.
-
Douaud G, Jbabdi S, Behrens TEJ, Menke RA, Gass A, et al. (2011) DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. NeuroImage 55: 880-890.
-
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338: 72-75.
-
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70: 687-702.
-
Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, et al. (2008) Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry 65: 1156-1165.
-
Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, et al. (2008) Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci 33: 423-430.
-
Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK (2012) Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14: 146-150.
-
Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, et al. (2013) Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: A longitudinal pilot study. Psychiat Res Neuroim 214: 197-203.
-
Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, et al. (2015) Activating positive memory engrams suppresses depression-like behaviour. Nature 522: 335-339.
-
Anderse P, Morris R, Amaral D, Bliss T, O'Keefe J (2006) The Hippocampus Book. New York, New York: Oxford University Press.
-
Peterson DJ, Ryan M, Rimrodt SL, Cutting LE, Denckla MB, et al. (2011) Increased Regional Fractional Anisotropy in Highly Screened Attention-Deficit Hyperactivity Disorder (ADHD). J Child Neurol 26: 1296-1302.
-
Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, et al. (2014) Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cerebral Cortex 24: 232-248.
-
Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85-93.
-
Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS (2009) Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 30: 3719-3735.
-
Lorenzetti V, Allen NB, Fornito A, Yücel M (2009) Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord 117: 1-17.
-
Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP (2002) Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry 180: 434-440.
-
Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, et al. (2006) Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry 12: 360-366.
-
Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W (2013) Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci 7: 2.
-
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44: 1105-1132.
-
Versace A, Almeida JC, Hassel S, Walsh ND, Novelli M, et al. (2008) ELevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry 65: 1041-1052.
-
Ross ED (2008) Sensory-specific amnesia and hypoemotionality in humans and monkeys: gateway for developing a hodology of memory. Cortex 44: 1010-1022.
-
Liao Y, Huang X, Wu Q, Yang C, Kuang W, et al. (2013) Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci 38: 49-56.





