International Journal of Allergy Medications
What is known about Oral Tolerance
Smolkin YS1,2* and Grishchenko EA2
1Department of Clinical Immunology and Allergology FGBOU DPO IPK FMBA, Moscow, Russian Federation
2LLC, Scientific Clinical Consultative Center of Allergology and Immunology, Moscow, Russian Federation
*Corresponding author:
Yury S. Smolkin, Department of Clinical Immunology and Allergology FGBOU DPO IPK FMBA, Moscow, Russian Federation, E-mail: smolkinyury@hotmail.com
Int J Aller Medcations, IJAM-2-013, (Volume 2, Issue 1), Short Review
Received: December 24, 2015: Accepted: March 25, 2016: Published: March 29, 2016
Citation: Smolkin YS, Grishchenko EA (2016) What is known about Oral Tolerance. Int J Aller Medications 2:013.
Copyright: © 2016 Smolkin YS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Healthy people are constantly exposed to foreign proteins but in contrast to allergic individuals they do not develop any immune response. Cornerstone of such difference is tolerance. Oral tolerance in allergic people is of highest priority.
Oral tolerance is specific suppression of cellular and/or humoral immune responses to an antigen by prior administration of the antigen by the oral route. It may develop naturally or be allergen immunotherapy (AIT) induced.
Mechanisms of natural tolerance development are not fully understood. Current data was mainly obtained from studies in patients, who receive AIT or have been exposed to high doses of allergens. Still it is not clear, why some allergic patients develop natural tolerance and some do not.
Oral tolerance is characterized by depletion or suppression of antigen-specific T-cells and induction of Treg-cells, which are clue cells in depression of inflammatory response to good antigens.
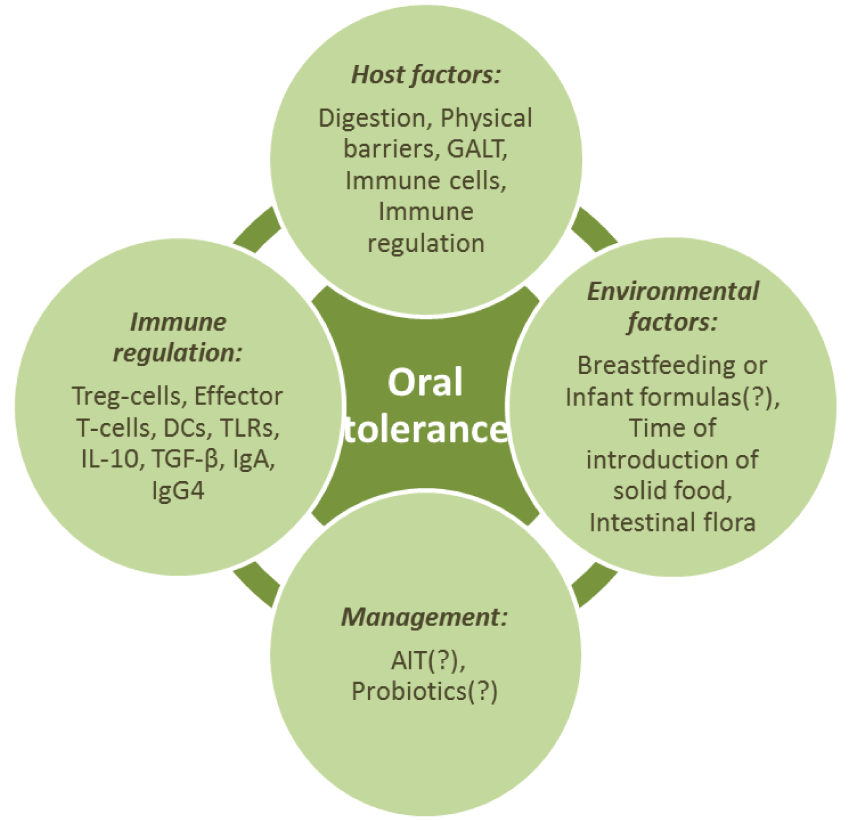
Oral tolerance to harmless dietary antigens develops due to different factors including antigen composition and dose, routes of antigen entry at sensibilization, regulatory T cells (Treg-cells) induction. Breastfeeding is also involved in tolerance development. Physical barriers, digestion, opportunistic pathogenic intestinal bacteria, gut-associated lymphoid tissue (GALT) immune cells, and immune regulation are supposed to be involved in oral tolerance development in the gastrointestinal tract.
In the present paper key factors and mechanisms involved in oral tolerance development as well as in food allergy development suppression are described.
Full understanding of oral tolerance mechanisms will help to reduce food allergy prevalence rates through preexposure prophylaxis (due to natural tolerance development) as well as to create new strategies of food allergy therapy (due to induced tolerance).
Keywords
Tolerance, Oral tolerance, Food allergy, Regulatory T cells (Treg-cells)
Introduction
Healthy people are continuously exposed to foreign proteins, but do not develop immune response, unlike people suffering from allergies [1]. The cornerstone of these differences lies in tolerance.
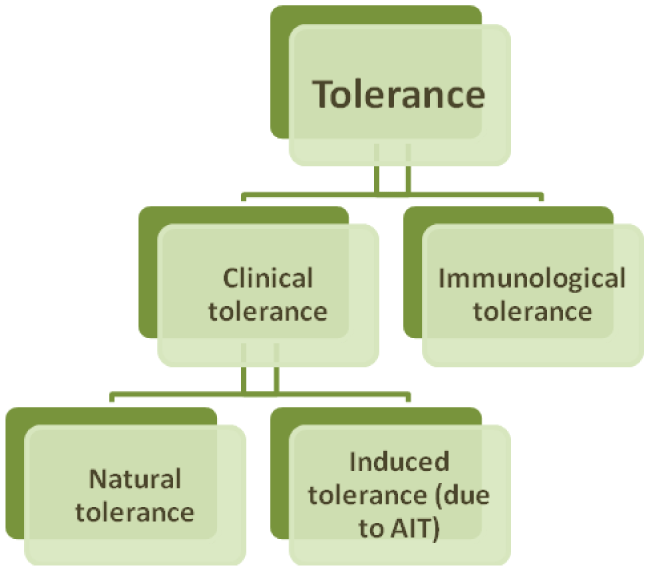
There are a few types of tolerance (Flow-chart 1). From the point of view of basic immunology, the term "tolerance" implies a condition of nonresponsiveness of adaptive immune system to a specific antigen. Development of immunological tolerance is important both for avoidance of autoimmune reactions and for prevention of immune response for innoxious environmental allergens or opportunistic pathogens [2].
Tolerance, determined as loss of responsiveness to antigen or allergen (clinical tolerance). It is a constant immunological condition where occasional or repeated exposure to antigen does not lead to development of allergic reaction [3]. For example, a person with tolerance to peanut will not develop clinical symptoms, despite the frequency and volume of consumption [2].
Although it is considered that clinical tolerance generally depends on immunological tolerance, their mechanisms may differ. The basis of clinical tolerance may lie in changes of innate and adaptive immune system, while mechanisms of immunological tolerance suggest involvement of adaptive immune system only [2].
Clinical tolerance may be natural or induced (e.g. through allergen immunotherapy (AIT)). Most people develop clinical tolerance naturally. Remarkably, natural tolerance may be associated with underlying hypersensitivity. Mechanisms of development of natural tolerance are not fully established. Existing data are mainly derived from studies of patients on AIT or exposed to naturally high doses of allergens (e.g. beekeepers and cat owners) [2].
An early description of the phenomenon of oral tolerance was provided by Wells and Osborne in 1911, when they described a series of studies showing that guinea pigs could not be induced to undergo experimental anaphylaxis to corn or oats if it was a component of the diet [4].
Oral tolerance may be determined as antigen specific suppression of cell and/or antibody-mediated immune response following oral antigen exposure [5]. Therefore, preliminary gastrointestinal exposure to antigen often leads to reduced reactivity to further local or systemic exposure to the same antigen [6].
In the intervening century, there has been a growing body of literature defining the immune mechanisms of oral tolerance. Classic oral tolerance experiments are performed by feeding of antigen, either a single high-dose feed or multiple low doses administered daily by gavage or in the drinking water for 5 to 7 days [4]. The number of studies addressing oral tolerance in humans is surprisingly limited despite the extensive literature from murine models. In fact, animal models have largely been used to study both the mechanism of sensitization to food as well as the resulting allergic response from consuming a food allergen. Thus, food allergy researchers have sought to develop an animal model that more closely mimics the sensitization of humans to food antigens. Until such a model, there may not be specific answers to the precise mechanisms that result in establishing oral tolerance or that lead to a break in tolerance [7].
Despite detailed immunological mechanisms underlying the immediate hypersensitivity reactions to food allergens are still studied, food allergy is likely to be the result of disrupted physiological development of oral tolerance or of disruption of already formed tolerance [8].
Two thirds of the children allergic to cow milk outgrow the allergy during the first year of their lives [9], by the age of three years the tolerance is developed in 85-90% of children [10]. On the contrary, only 22% of children diagnosed with peanuts allergy by the age of 1 year develop the tolerance by the age of four [9].
Why do some patients with allergy develop natural tolerance, while others don't, is not clear, but induction of allergen-specific regulatory T cells (Treg-cells) and decreased production of specific IgE may be involved in the process [2].
In this article we consider the key factors and mechanisms involved in oral tolerance relative to development of food allergy.
Mechanisms of Oral Tolerance
Initially, oral tolerance mechanisms were explained through peripheral tolerance mechanisms, namely clonal deletion and a nergy of antigen-specific T-cells, caused by insufficient antigenic stimulation, usually in the absence of colony stimulating signals. However, such mechanisms would not explain how oral tolerance may be transferred through adoptive transfer of immune cells from tolerant mice to recipients earlier exposed to those antigens. The investigational approach revealed an alternative mechanism of oral tolerance. It was shown that population of CD4+ Т-cells, normally constantly expressing CD25, has regulatory properties [6].
T-cell unresponsiveness or anergy is one of the primary mechanisms by which tolerance is maintained in self-reactive lymphocytes. The upregulation of anergy-associated genes is largely dependent on nuclear factor of activated T cells. Orally tolerized T-cells can form conjugates with antigen-presenting cells, but they are defective in immunologic synapse formation [7].
Development of oral tolerance to food allergen implies early changes in intestinal mucosa. Originally there is a need for forming local immunosuppressive intestinal environment, conditioned by immunomodulatory cytokines, such as IL-10 and TGF-β, providing non-specific inflammation control. Further, the medium promotes development of regulated systemic immune response and differentiation of antigen-specific CD4+CD25+FохP3+ Treg-cells required for immune homeostasis in intestine [11].
Oral tolerance is characterized by deletion or suppression of antigen-specific T-cells and production of Treg-cells inhibiting inflammatory response to benign antigens [12]. Clonal deletion and anergy of T-cells seems to be less relevant in the context of food allergy than regulation by Treg-cells [13].
The balance of effector and Treg-cells in the gut appears to regulate the tolerance to food allergens. Tolerogenic condition in respect to food antigens in healthy people is characterized by prevailing of Treg-cells in lamina propria of intestinal mucosa. That is why lack of effects of induced Treg-cells (iTreg-cells) in mucosal lamina propria causes development of food allergy [3].
One interesting experimental system that has been used to study T-cell function in oral tolerance is the use of TCR transgenic mice, in which all T-cells have a common TCR. Using such mice, Dr Weiner and colleagues investigated how oral administration of an antigen affected specific T-cell subsets. These investigators showed a dose-dependent induction of Treg-cells to the fed antigen [7].
Tolerance developed after exposure to high doses of allergen is thought to be related to induction of IL-10 producing Treg-cells and production of inhibiting allergen-specific IgG4 [2]. Also it was shown that high doses of allergen contribute to induction of anergy or deletion. In fact, clonal deletion was found in the periphery but not the thymus, suggesting that high-dose oral tolerance not only induces deletion but may lead to CD4+CD25+ Treg-cells that resemble natural Foxp3 Treg-cells [7].
Development of tolerance to low antigen doses may be related to various mechanisms, such as extrathymic expression of FoxP3 transcription factor by Treg-cells [2].
Antigen composition plays a significant role in development of tolerance: soluble antigens are more tolerogenic versus undissolved antigens [8].
Immune mechanisms of tolerance may vary depending on the site of exposure to allergen. Immunosuppressive TGF-β cytokine is required to develop tolerance induced on the surface of mucosa, not skin [2].
One of studies has shown that clinical improvement of symptoms in patients with chicken egg allergy was associated with growth of specific IgG4 and reduction of specific IgE to OVA. In children with allergy to milk and/or eggs, low level of IgG4 to OVA and/or β-lactoglobulin evidenced the need for maintaining long-term elimination diet. The study comparing non-atopic patients to patients with cow milk allergy, it was reported that maintaining tolerance to cow milk protein was associated with a higher level of specific IgG4 to cow milk [5].
Specific IgG4 acts by inhibiting of the binding specific IgE to allergen [5]. In patients allergic to peanuts it was noted that allergen-specific IgG4 play role in inhibition of CD23-mediated presentation of allergen by B-cells to T-cells [3]. Upregulation of allergen specific IgG4 production induced by IL-10 is, however, also related to the development of tolerance [5].
Specific IgG4-response to food antigens can be physiological as the result of continuous exposure to antigen [5].
Regulatory Т-cells (Treg-cells)
Treg-cells deserve particular attention while discussing mechanisms of tolerance, including oral tolerance. It was shown that inefficient induction of Treg-cells contributes to the development of food allergy [8].
Weiner and colleagues first described a population of cells termed Th3-cells, that express TGF-β. These cells do not express CD25 or Foxp3, and are suppressed by a TGF-β–dependent mechanism. In addition to these cells, antigen-specific Treg-cells expressing the transcription factor Foxp3 are also induced in response to antigen feeding, and these iTreg-cells also suppress through a TGF-β–dependent mechanism. Tr1-cells that are IL-10 dependent and suppress through an IL-10–dependent mechanism are involved in the prevention of colitis and microbial-induced inflammation in the intestine [4].
Transcription factor Foxp3, expressed by Treg-cells is necessary for their development and for enhancement of suppressor function [6]. FoxP3 directly interacts with GATA-3 and so inhibit expression of cytokines activated by GATA-3 (IL-4, IL-5, IL-13) [5].
FoxР3-mutant mice, DEREG-mice with ability to inhibit FoxP3 expression by diphtheria toxin, and patients with IPEX-syndrome (immunoregulatory X-linked polyendocrinopathy and enteropathy syndrome) with FoxР3 genetic locus mutation demonstrated the significance of FoxР3+Treg-cells in development of tolerance [1].
Foxp3+Treg-cells may be formed both in thymus and outside peripheral lymphoid organs by differentiation of normal mature CD4+ Т-cells at specific conditions. Considering that Foxp3+ Treg-cells may be divided into two types: of thymic (tTreg-cells) and peripheral (pTreg-cells) origin, with pTreg-cells more important for oral tolerance [6]. De novo development of Treg-cells in GALT is a significant condition of development of oral tolerance [5].
Using a murine model to examine the role of the thymus in high-dose oral tolerance, researchers found that thymectomized animals were not protected from autoimmune disease. The thymus was actually found to be an important site for the development of CD4+CD25+ Treg-cells after oral antigen [7].
In fact, all major classes of Treg-cells can be induced or activated by oral antigen. Even CD8+ Treg-cells have been shown to play a role in oral tolerance [7].
Although Treg-cells specific to food allergens are formed and localized in intestine, they also can be found in circulation (particularly with allergen exposure) to maintain systemic tolerance [5].
The underlying mechanisms behind the unique function for pTregs remain largely elusive [6]. Most of knowledge about Treg-cell activity in the context of allergic disorders, has mainly been obtained from AIT studies, not natural tolerance [14].
Foxp3+Treg-cells utilize different sets of mechanisms to maintain tolerance. They may produce inhibiting cytokines, like TGF-β, IL-10 and IL-35, express granzymes for induction of direct cytolysis of effector T-cells, inhibit IL-2 for effector T-cell proliferation or inhibition and/or modulate maturation or function of DCs [6].
TGF-β is one of the primary molecules inducing and maintaining Treg-cells. Secreted and cell surface–associated forms of TGF-β suppress activation of effector T-cells in intestinal inflammation [15]. TGF-β inhibits activity of Т- and В-cells and activates production of secretory IgA [1].
Using murine food allergy models receiving Staphylococcus' enterotoxin B with OVA or peanut, Ganeshan et al. have shown that Staphylococcus' enterotoxin-В inhibited expression of TGF-β and Treg-cells and activated response to peanut antigens inhibiting induction of tolerance [8].
Treg-cells produce IL-10 at high concentrations [12]. IL-10, inducing anergy of effector T-cells, at the same time maintains population of Treg-cells and participates in activation of switching B-cells to production of secretory IgA [1]. In vitro IL-10 may lead to B-cell production, related more to IgG4 production, rather than to IgЕ production. The potential role of IL-10 in recovery from milk allergy (and other food) was indirectly supported in many studies. Most data support the significance of IL-10 secretion by Tr1-subtype of Treg-cells for development of tolerance [14]. Gri et al. demonstrated that Treg-cells directly inhibit degranulation of mast cells through intercellular contacts and IL-10 production [12].
It is well known that the cytokine IL-10 is necessary for the maintenance of immune homeostasis in the gastrointestinal tract, and in the absence of IL-10 there is the development of spontaneous colitis from inappropriate immune reactivity to the commensal microbiota. However a number of studies shows that IL-10 is dispensable for induction of tolerance to dietary antigens. The fact that IL-10 is critical for tolerance to the microbiota but dispensable for oral tolerance to fed antigens suggests that different immunoregulatory mechanisms are responsible for tolerance to foods compared with microorganisms [4].
TGF-β-induced iTreg-cells were shown to be "flexible" in differentiation in specific cytokine environment and may differentiate to proinflammatory Тh17-cells. Тh17-cells in presence of certain cytokines may differentiate into effector Тh1- and Тh2-cells. Therefore, microenvironment of mucosal lamina propria plays a key role in the life of naive CD4+ Т-cells controlling the balance between regulatory and proinflammatory T-cells and development of tolerance or proallergic condition [3].
It is quite possible, that Foxp3+ Treg-cells adjust their suppressive mechanisms to environmental conditions [6]. High doses of antigens lead to predominant anergy of T-cells, but may also induce switching of Th1- and Th2-cells to secreting IL-10 Tr1-subtype of Treg-cells. Low doses of antigen usually activate other types of Treg-cells [5].
Qamar and colleagues chose to investigate the role of Treg-cells in the egg or peanut-allergic children spontaneously acquiring tolerance to hen's egg white or peanut. As the result of the study the authors hypothesized that the increased CD3+CD4+CD25+CD127lo+ cells at baseline and upon stimulation and increased induction of IL-10-producing cells of several types, including Tr1-cells, from naturally tolerant patients suggest an important role for regulatory T-cell subsets in the acquisition of natural tolerance [13].
Karlsson et al. have specified that children who finally "outgrew" milk allergy had higher levels of circulating CD4+CD25+ Treg-cells [8].
Shreffler et al. demonstrated that milk protein specific FoxР3+CD4+CD25+ Treg-cells were detected at high levels in children who developed tolerance to heated milk versus those who had milk (including heated milk) allergy maintained, and healthy children from the reference group. It was shown that higher levels of allergen-specific Treg-cells correlated to milder clinical course of food allergy to milk and more positive prognosis. Certain observations and results of previous longitudinal research confirm that children tolerating heated milk outgrow allergy sooner than children with allergic response to heated milk [14].
Dang et al. have shown that the nature of allergen hypersensitivity is related to Treg-cell deletion after exposure to allergen. In children without symptoms of allergy stable levels of Treg-cells were observed for a long period after challenge. Hypersensitive children experienced decrease of Treg-cell levels with recovery to baseline at day 6. Children with food allergy demonstrated consistent reduction in the number of Treg-cells documented after exposure to allergen. Besides, children with food allergy had significantly lower levels of activated Treg-cells. Weakened ability to regenerate Treg-cells after exposure to allergen may be a significant factor that might explain differences between clinically manifested allergy and asymptomatic hypersensitivity [16].
Yamashita et al. studied mechanisms of oral tolerance induction in a murine food allergy model. For that purpose the available murine model of food allergy was modified by administration of OVA or transfer of cells from mesenteric lymph node or of T-cells obtained from mice exposed to OVA. Prior exposure to OVA provided certain prevention of food allergy, completely inhibiting OVA-specific IgE, IgA production, and IL-4, IL-9 and IL-10 expression. The number of Treg-cells among mesenteric lymph node and TGF-β expression was increased. Through transfer of mesenteric lymph node cells and Treg-cells from mice after prior exposure to OVA, the transfer model demonstrated inhibition of anaphylaxia in response to OVA administration. However, through transfer of antigen-specific and non-specific Treg-cells of mice after prior administration of OVA, expression of OVA-specific IgЕ and IgА was partially weakened. In the model of Treg-cell transfer expression of IL-4 and IL-10 decreased, while expression of IL-9 increased. It was suggested that Treg-cells related to IL-9 production indirectly affect acquired tolerance through differentiation and degranulation of mast cells. In conclusion, authors of the study have found that oral tolerance to food allergens is induced in two ways: through prior exposure to antigen (congenital tolerance) or through transfer of Treg-cells (acquired tolerance). As food allergy develops when there is no congenital tolerance, understanding of the nature of acquired tolerance is important, as it may help to develop new ways of treatment of food allergy [15].
Host Factors that Influence Oral Tolerance
Physical barriers, digestion, certain cells of immune system and immune regulation are also thought to contribute to the ability to develop oral tolerance in the gastrointestinal tract [1].
Digestion process, physiological barriers, gut-associated lymphoid tissue (GALT) will be reviewed in the next section. Immune system cells, the most important of which for oral tolerance are the Treg-cells, effector T-cells, DCs, and immune regulation, performed with the abovementioned cells, special receptors (TLRs), cytokines (IL-10, TGF-β), immunoglobulins (IgA, IgG4), are discussed in different sections of the review.
The Role of Gastrointestinal Tract in Development of Oral Tolerance
Digestion is also important for forming oral tolerance [1]. Usually, we digest over one hundred grams of food proteins daily [6]. Gastric acid and gastrointestinal enzymes help digesting food proteins enabling absorption of nutrients. That process leads to decreased number of epitopes of food proteins that break up into less immunogenic dipeptide and tripeptide chains [1].
The role of gastric acid in tolerance development is supported by the fact that using acid neutralizing drugs for ulcer treatment triggers production of IgE to food allergens. Further studies in animals have shown that changes in gastric pH are important for IgE production and lead to changes in gastric epithelium in mice, resembling human's food allergy [1].
Potential role of concomitant use of gastric acid inhibitors, lipids, antioxidants or vitamins and sensitizing antigen in oral tolerance induction (through modulation of allergen processing and activation of immune system cells) is of particular interest and is a subject of research [8].
Intestine is the largest immune organ in the body. For the most part it responds to pathogens, develops tolerance to innoxious exogenous antigens and maintains opportunistic bacterial flora [5]. The greatest antigenic burden from food occurs in the small intestine, whereas the greatest antigen burden from the microbiota occurs in the colon [4]. The process of absorption occurs along the full length of small intestine, but about 50% of proteins are absorbed in duodenum [12].
Intestinal barrier and its failure play a central role in development of oral tolerance. In neonatal period, intestinal barrier is immature and is characterized by increased permeability for macromolecules. Increased intestinal permeability may impact development of tolerance because of increased antigenic load [5].
In one of the studies, the onset of peanuts allergy before the age of 14 was related to the presence of specific IgE to such components of peanuts as Ara h 1, Ara h 2 and Ara h 3. Patients with later onset of the disease were more sensitized to the peanuts component having the crossover structure with pollen (Ara h 8) or with food of plant origin (Ara h 9). Precise mechanism underlying those differences is unknown. It is presumed that the disruption of intestinal barrier in children with genetic predisposition to allergies leads to increased intestinal permeability and contributes to the development of sensitization to reserve proteins [9].
Barrier function of gastrointestinal tract is performed through defensive hydrophobic mucous surface and secretory IgA [1]. Tight junctions between intestinal epithelial cells, presence of antimicrobial peptides in mucosa secreted by certain cells of intestinal epithelium help to maintain the barrier function [6].
The layer of muco-oligosaccharides promotes capture of antigens, while secretory IgA binds food proteins and prevents antigenic absorption through intestinal epithelium [1]. Antigen-specific IgA produced via TGF-β, is linked to development of tolerance, probably through activation of IL-10 production [5].
Relatively high prevalence of allergy in patients with IgA deficiency supports the protective role of IgA against allergy and/or tolerance development. It is reported, that high level of IgA in intestine of infants is associated with lower risk of IgE-mediated allergy [5].
Treatment with specific IgА demonstrated inhibition of anaphylactic reactions induced by food antigens [15]. One of the studies, assessing protective effects of mucosal IgA against food allergy, demonstrated that mice with induced polymer immunoglobulin receptor deficiency (involved in the process of IgA secretion into the intestinal lumen) were hypersensitive to IgG-mediated anaphylaxis. Strait et al. suggested that it was systemic, and not secretory IgA, that played the major role in protection against IgE-mediated anaphylaxis [1].
GALT is the primary pathway of allergen effects. In case of food allergy, evidence of other ways of hypersensitivity was presented. Probably, primary extraintestinal interaction of food proteins to immune cells may lead to inability to form oral tolerance [3].
Human GALT contains a large number of cells of innate and adaptive immune system (1012 lymphoid cells per 1 m2 of small intestine) [6].
It is suggested that antigen uptake is a key critical point of successful induction of oral tolerance [5]. Processing of food proteins in gastrointestinal tract involves three types of targeted cells, including M-cells, epithelial cells and DCs. All these play an important role in antigen presentation and oral tolerance development [1].
Enterocytes may play a key role in uptake of soluble antigens and activation of CD8+ T-cells with suppressive activity. It was suggested that enterocytes regulate the rate and method of antigen absorption [12].
Recently, a novel mechanism of small intestinal antigen uptake was identified through goblet cell–associated antigen passages (GAPs). A conduit was identified in small intestine that rapidly filled with luminal antigen, delivering antigen to lamina propria DCs. Intestinal mononuclear phagocytes have been shown to extend dendrites between enterocytes, reaching into the lumen and pulling antigen across the epithelium without disrupting the integrity of the tight junctions between cells. This mechanism of antigen uptake was under the control of cholinergic regulation, showing an important point of control of mucosal immunity by nerves in the gastrointestinal tract [4].
Antigen presenting cells (primarily DCs) participate in processing of food allergens and their presentation through MHC class II receptors to T-cells finally leading to development of oral tolerance through inhibition of antigen-specific T-cells and induction of Treg-cells, which inhibit inflammatory response to antigen [12].
Gastrointestinal mucosa contains a few various populations of DCs involved in processing and presentation of antigens and playing a role in development of tolerance [1].
Significance of intestinal DCs for development of oral tolerance primarily was based on the fact that increased number of DCs through Flt3 ligand activity increases effectiveness of oral tolerance. Further studies have shown a certain role of CD103+ DCs in processing and presentation of food antigens to T-cells. Intestinal DCs continuously migrate to mesenteric lymph nodes involving CCR7. CCR7 deficient mice had impaired ability of CD103+ DCs in lamina propria to migrate to mesenteric lymph nodes, making it impossible to induce oral tolerance. Surgical excision or immaturity of mesenteric lymph nodes prevents oral tolerance, enabling us to suggest that migration of CD103+ DCs from lamina propria to mesenteric lymph nodes is a significant factor contributing to oral tolerance [6].
CD103 integrin expressing DCs (CD103+ DCs), have tolerogenic properties. After exposure to antigen in mucosal lamina propria and migration to mesenteric lymph nodes, they produce TGF-β and retinoic acid, activating FoxP3 synthesis by naive CD4+ T-cells and their differentiation into induced Treg-cells (iTreg-cells). Conversely, another population of DCs (CD103- DCs) is characterized by pronounced proinflammatory activity and induces differentiation of naive CD4+ T-cells into Тh1/Тh17-cells [3].
It is generally accepted that DCs of Payer's patches are more important for detection of large particle antigens, transported by M-cells, such as intestinal bacteria and viruses, rather than soluble food antigens [6].
Research is ongoing with the purpose to demonstrate manipulations with DCs enabling improvement of Treg-cell function and/or affect the balance of Тh1/Тh2-cells and launch development of tolerance to food antigens [12]. Some studies have shown protective effect of CD103+ DCs for food allergy through the exposure to superantigen or prolonged exposure to oral antigen [17].
Yang et al. studied inhibition of allergic reaction in β-lactoglobulin sensitized mice through regulatory DCs induced by Lactobacillus paracasei L9. Addition of Lactobacillus paracasei L9 restored the impaired Th1/Th2-cell balance in mice with reactions to β-lactoglobulin through activation of CD4+CD25+FoxP3+ Treg-cells. Moreover, administration of Lactobacillus paracasei L9 significantly induced expression of CD103 and reduced maturation of DCs in mesenteric lymph nodes, Peyer patches and spleen. DCs from bone marrow in vitro were activated by Lactobacillus paracasei L9 with approximately 1.31-fold and 19.57-fold increase of CD103 CD11c+ DCs expression and production of IL-10, respectively, while expression of CD86 did not significantly change. The data obtained demonstrate that Lactobacillus paracasei L9 reduces sensitization to β-lactoglobulin, probably, through enhancement of suppressive activity of regulatory DCs [17].
The intestinal mucin Muc2 has been shown to act as a tolerogenic adjuvant promoting the development of regulatory T cells to co-administered antigens. Muc2 interacts with CD103 DCs through a receptor complex. Ligation of this receptor complex results in β-catenin signaling that suppresses inflammatory NF-κB signaling. Muc2 enhances the regulatory phenotype of CD103 DCs by increasing the expression of TGF-β and RALDH. In the absence of Muc2, tolerance is impaired to fed antigens; exogenous mucin can restore the development of tolerance in Muc2-/- mice [4].
The role of different organized lymphoid structures in the development of oral tolerance has been addressed by several studies, the results of which suggest that PP are dispensable for the induction of oral tolerance, whereas mesenteric lymph nodes are required [4].
Except for intestine, the other potential place for development of oral tolerance may be the liver that has several features that could serve to maintain the tolerance. Administration of antigen directly into the portal vein, which drains blood from the intestine to the liver, is well known to induce antigen specific tolerance. Conversely, directing blood flow away from the liver by portocaval shunting prevents the induction of oral tolerance [7].
Environmental Factors and Oral Tolerance
Breastfeeding
Advantages of the breastfeeding regarding the stimulation of immune system and proper development of intestinal barrier are generally accepted [18].
Breast milk serves as a buffer to keep the baby's gastrointestinal tract at a higher pH, allowing greater absorption of nutrients and survival of bacteria in the lower gut. Human milk is additionally composed of a human milk microbiota (primarily Proteobacteria and Firmicutes) and prebiotic components (such as human milk oligosaccharides) that stimulate bacterial growth and are bifidogenic, as well as antimicrobials (such as IgG, IgM and secretory IgA). Recent works suggest that it is not the initiation of solid food but the cessation of breastfeeding that begins to shift the infant's gut microbiota to an adult pattern [19].
There are still many disputes regarding the role of breast milk components in the process of development of allergies [18].
TGF-β and IL-10 are tolerogenic cytokines detected in breast milk. In 2008, TGF-β was shown to play a significant role in breast milk–induced tolerance, mediating CD4+ lymphocytes [20].
IgA is the major antibody of breast milk inversely related to atopic dermatitis. In mothers of atopic children the total protein level in breast milk was higher [20].
The relation between fatty acids of breast milk and development of allergies is widely studied. Most likely, n-3 polyunsaturated fatty acids (n-3 PUFAs) have protective features, while consumption of large quantities of n-6 polyunsaturated fatty acids (n-6 PUFAs) increases the risk of development of allergies. One of the studies showed that the relation between presence of fatty acids in breast milk and development of sensitization, eczema and asthma is present not only in early age but up to the age of 14. This particular pattern was observed regardless of the presence of allergy in mothers of studied children [21].
One of the studies of food allergy in OVA-sensitized mouse model studied the role of two human milk oligosaccharides (2'-fucosyllactose and 6'-sialyllactose). As a result it was shown that the oligosaccharides reduced the symptoms of food allergy via induction of IL-10+ Treg-cells and stabilization of mast cells. Prebiotic nature and increasing number of proof of immunomodulating properties of breast milk oligosaccharides allow us to presume the presence of a certain therapeutic potential of the latter regarding allergy [22].
Using the microarray assay we showed the presence of allergens in breast milk which is likely related to the diet of mother [18]. This fact allows us to explain the development of cow milk allergy in breast-fed children. Such peanuts allergens as Ara h 2 and Ara h 6, likewise cow milk allergens, are transferred via breast milk being immunologically active [9].
Recent simulation in mice confirmed the theory that breastfeeding reduces the risk of allergy. In 2011, one of studies showed that the transfer of antigen and antibody in breast milk led to tolerance, the results of which were similar to those of a study showing oral tolerance in pups of aerosol-sensitized mothers exposed to allergen [20]. In this particular study transfer of antigen-IgG immune complex to the neonates via the breast milk of sensitized mothers led to induction of antigen-specific FoxP3+CD25+ Treg-cells. Induction of oral tolerance with immune complexes of breast milk did not require milk to contain TGF-β, unlike the tolerance induced by free antigen transferred via milk. The study highlights that IgG breast milk immune complexes are potent inducers of oral tolerance [23].
In 2012, review further supported breast milk as being protective against allergy [20].
However, one of recent studies in children living in urban area, whose parents suffered atopic disorders, have shown that breastfeeding of any duration was significantly associated with food allergy [20].
Understanding of interrelation between food allergy and breastfeeding may give rise to development of new areas of research of allergy prevention methods [20].
Infant formulas
According to some studies, in cases when breast feeding for infants with high risk of allergies development is impossible the use of extensive hydrolysates (eHF) or partial hydrolysates (pHF) and avoidance of standard cow's milk-based formulas (SF) during the first 4 months of life can be useful regarding the prevention of allergy development [9].
It is thought that hydrolyzed formulas have immunomodulating properties. So far there is indeed increasing in vitro evidence that hydrolysates contain specific immunomodulating peptides. They have been found to improve the epithelial barrier, modulate the Th1/Th2 balance and the amount of Treg-cells towards a less Th2 skewed response, and decrease inflammation, which is all beneficial in food allergy. The limited in vivo studies available confirm these findings so far. However, making general statements about the hypoallergenic and immunomodulating effects of hydrolysates is not possible since every hydrolysate is unique. For example, cow's milk proteins digested with pancreatin or trypsin were found to inhibit lymphocyte proliferation, while digestion with pepsin or chymotrypsin did not cause these effects [24].
Results of one of the recent studies showed that the addition of docosahexaenoic acid and arachidonic acid in infant formulas increases protection from allergy in early childhood [25].
In cases of cow's milk allergy and when breast feeding is impossible it is recommended to prefer hydrolysed formulas, while the amino acid based formulas (AAF) are restricted for the most severe cases. Rice hydrolysates and soy formulas are the alternative variants. Addition of prebiotics and probiotics to eHF (L. rhamnosus GG, Bifidobacteria breve) may bring additional benefits [10].
Time of introduction of solid food
The time of introduction of solid food is one of the factors affecting development of oral tolerance. Delay of intestinal colonization or late antigen exposure may lead to inability to develop an oral tolerance. However, too early antigen exposure when intestinal colonization is not yet complete and local immune system is not yet developed, may increase the risk of allergy or an autoimmune disease [3].
Modern studies are aimed at supporting the hypothesis that early systematic exposure to food allergens (versus exclusion or delayed introduction into diet) with higher probability may lead to induction of oral tolerance and decreased risk of food allergy [26].
A number of studies on human population showed that the inclusion of milk, eggs, fish and oats in the diet at the age older than 6-9 months is associated with increased risk of development of atopic dermatitis and allergies [27].
In the process of oral tolerance development there is a critical time interval where a risk of allergy may be reduced. Although the time limits are not fully known, recent studies have demonstrated that they fall within Month 4 and Month 7 of life [3].
One of the particularly curious conclusions of the STAR trial was the following observation: in significant part of children with eczema from the high risk group food sensitization and clinical symptoms developed before the introduction of solid food in the diet. The fact indicates that the processes leading to food sensitization are well developed in some children at this age, which makes necessary to establish earlier preventive interventions [26]. Most current randomized, controlled studies assess the optimal period of introduction into diet of chicken eggs and peanuts [26]. Koplin et al. (2010) demonstrated that infants who began to consume eggs at month 4-7 were at the lowest risk of egg allergy [3].
In countries where peanut containing snacks are approved for pediatric use, the frequency of peanut allergy is lower. Du Toit has shown that the prevalence of peanut allergy (despite apparently early introduction into the diet) among Jewish children in Israel was 10-fold lower versus children in UK with similar genetic background [8]. LEAP trial also showed that early introduction of peanuts significantly decreases frequency of peanuts allergy in children in high risk group [28].
Are there any differences in the diet of children with food allergy and without it?
In case of food allergy development the main therapeutic action is the elimination diet [9]. One of the studies showed that the elimination diet did not affect the growth of children with food allergy if the diet of the child was adequately supplemented with other products that were not forbidden [29].
The time of introduction of solid food in the diet of the patients with food allergy and without it shall not be different [30].
Intestinal flora
Human intestinal microbiota numerically exceeds human host cells approximately 10-fold, and what is more important, has approximately 100-fold genetic diversity [6]. It is highly probable, that opportunistic intestinal flora is involved into the oral tolerance [3].
It was observed, that mice grown in sterile environment do not develop normal tolerance. In murine peanut allergy models it was shown that TLR4-deficient mice receiving antibiotics to inhibit intestinal flora were more susceptible to peanut allergy, versus control animals without mutations [3].
In the era of industrialization reduction of microbial exposure at early stages of life may lead to deregulation of T-cells presenting as induction of allergic inflammation [12].
Use of antibiotics can lead to alteration of intestinal microbiota in the children of first year of life, which can affect the immune system. Penders et al. found that the use of antibiotics leads to decreased numbers of Bifidobacterium and Bacteroides [19].
TLRs recognize specific markers on the surface of intestinal flora bacteria, so called PAMPs (Pathogen-associated molecular patterns). Some TLR agonists may activate Treg-cells, while others can trigger hypersensitivity. Research of probiotic bacterial strains protecting against food allergen hypersensitivity is of special interest [12].
TLR2 is a key mucosal immunity regulator. TLR2 activators are found in many types of food. Tunis et al attempted to assess effects of expression and activation of TLR2 on oral tolerance to food allergens in a murine model. Mice received OVA or peanut butter with/without low doses of TLR2 activators (PAM3CSK4 or FSL-1). The authors concluded that TLR2 is not an obligatory component for induction of oral tolerance, but oral activation of TLR2 modulates antibody induced immunity for development of tolerance (through IgЕ and IgА). Low dose of PAM3CSK4 is also an effective oral adjuvant and selectively increases IgA production. Such observations may be used for optimization of oral AIT and vaccine development [31].
Some Lactobacillus and Bifidus strains demonstrated effects on immune response through different immunological mechanisms acting on enterocytes, antihypertensive cells, TLR2, TLR4, TLR9, Treg-cells and effector Т- and В-lymphocytes. Opportunistic intestinal bacteria inhibit local inflammatory reactions. The intestinal microbiota also promotes the production of TNF-α and PGE2 that interfere with the development of tolerance mediated by DCs. Probiotics were shown to polarize the immune response in the Th1-cell direction under DCs effects. Intestinal flora also affects IgA production in the distal part of small intestine [12].
In animal studies probiotic supplements induced Treg-cell production. In vitro studies have shown increased production of IL-10 in humans after treatment with Lactobacillus Reuteri and Casei, but not Plantarum. Lactobacillus Reuteri and Casei were shown to stimulate DCs enhancing Treg-cell production. Lactobacillus Reuteri and Casei can bind intercellular lecithin-like adhesion molecule specific for DCs, thus inhibiting its potential contact with antibodies [12].
In one of studies of OVA allergy Lactobacillus rhamnosus was shown to increase the number of CD4+CD25+FoxP3+ Treg-cells and enhanced secretion of TGF-β in mesenteric lymph nodes, but not in spleen [17].
AIT induction of oral tolerance
In cases when the natural tolerance is not formed or the food allergy has developed there is the a question "How is it possible to induce the tolerance?"
AIT can cause the development of tolerance to food allergens including sublingual (SLIT) and oral (OIT) immunotherapy [9].
First case of applying OIT for treatment of child allergic to eggs with severe cases of anaphylaxis was reported by Lancet in 1908. Early studies of subcutaneous allergen-immunotherapy (SCIT) with peanuts were terminated due to high risk of anaphylactic reactions. Recent studies of OIT or SLIT with peanuts, milk and eggs showed some positive prospects. OIT that uses raw or baked products is more effective than SLIT. Nevertheless, the systemic reactions requiring adrenaline use were observed in 25% of participants, especially when raw products were used, which prevented performing OIT in daily practice. The concomitant use of omalizumab can decrease the incidence of adverse reactions and increase the efficiency of AIT [32].
While AIT is accompanied by increased level of allergen-specific IgG and decreased activation of basophiles, currently there are no biomarkers for prediction of possible reactions on food. Efficiency of AIT can be displayed only while using of oral provocation tests [32].
Also there is still no clarity on the necessary duration of OIT, also it is uncertain whether OIT leads to the development of oral tolerance or only to desensitization [32].
Due to the risk of adverse reactions including anaphylactic shock European Academy of Allergy and Clinical Immunology (EAACI) does not recommend AIT for routine clinical use in the treatment of food allergy (level III, class D). AIT shall be performed only in specialized centers with experienced personnel and necessary equipment in accordance with the protocols approved by the local ethics committees [32].
Dietary Recommendations
Taking into account the protective action of a number of substances regarding food allergies, there is the question about dietary recommendations for pregnant and lactating women, as well as infants.
Limited consumption of allergen products by the mother during pregnancy for the primary prevention of food allergy (including allergy to milk and eggs) in the early childhood did not show the protective effect [27]. In particular, despite the transfer of maternal IgG, one of the studies displayed that the consumption of peanuts pre- and post-partum did not affect the sensitization to peanuts and induction of oral tolerance in offspring, which confirmed the lack of necessity of dietary limitations in pregnancy and lactation for prevention of food allergy [33].
Zeiger and Heller noted that by the age of 7 the children whose mothers excluded from their milk, eggs and peanuts in the third trimester of pregnancy, and who avoided the consumption of milk till the age of 1 year, consumption of eggs – till 2 years, of peanuts and fish - till 3 years, had no differences in prevalence of food allergies and any atopic diseases with control group which had no nutritional limitation [27].
The effect of n-3 PUFAs containing additives in the diet of pregnant women and infants on the prevention of allergies is actively studied. Despite the controversial results, the majority of studies display the protective effect [21]. In the United Kingdom, in order to achieve the optimal consumption of n-3 PUFAs, the pregnant women are recommended to consume two portions of fish per week, at least one of which should contain fatty fish [34].
While several meta-analyses showed the advantages of probiotics in eczema prevention, it is difficult to turn the received results into clinical recommendations. International expert organizations do not recommend the routine use of probiotics for allergy prevention, however, recently World Allergy Organization (WAO) and McMaster University have begun the development of guidelines for probiotics as a preventive measure for allergy. In their report they noted that the existing data do not confirm that probiotics reduce the risk of development of allergies, but it is likely that they have some advantages (mostly for eczema prevention). In case of healthy people it was proposed to review the use of probiotics in pregnant and in lactating women, in women with high risk of giving birth to an allergic child based on family anamnesis, in infants in case of high risk of allergies development. It was stressed that the guidelines are conditional and based on low evidence base, which also complicates the creation of more specific recommendations [35].
Conclusion
Oral tolerance development is a complicated multicomponent (Flow-chart 2) and really significant process, which under tense antigenic impact allows to suppress inflammation response towards harmless antigens coming with food.
Basically, development of oral tolerance is provided by adhering to balance between effector T-cells and Treg-cells. In case of steady growth of food allergy prevalence the answer to the question of how to preserve or, if necessary, to obtain this balance is of the utmost importance in view of food allergy prophylaxis and treatment. However, it is also let the interference aiming at tolerance induction effect bring along a reverse effect which is suppression of immune response to insecure antigens coming into gastrointestinal tract.
From our point of view, studies of Treg-cells, DCs, TLRs induction methods and use of various substances (including probiotics) capable to induce oral tolerance and prevent sensibilization are extremely perspective.
When creating favorable conditions for induction of oral tolerance, one shall adhere to certain recommendations based on the results of modern studies (Table 1).
![]()
Table 1: Recommendations for creating favorable conditions for induction of oral tolerance.
View Table 1
Complete understanding of oral tolerance mechanisms will help to resolve the most significant problem of food allergy prevalence reduction by means of primary prophylaxis (via natural tolerance development), as well as to create new strategies for food allergy treatment (via induced tolerance).
References
-
Pelz BJ, Bryce PJ (2015) Pathophysiology of Food Allergy. Pediatr Clin North Am 62: 1363-1375.
-
Timothy P. Moran, Wesley Burks A (2015) Is Clinical Tolerance Possible after Allergen Immunotherapy? Curr Allergy Asthma Rep 15:23.
-
Cabrera CM, Urra JM (2015) Food allergy and the oral immunotherapy approach. Arch Immunol Ther Exp (Warsz) 63: 31-39.
-
Berin MC, Shreffler WG (2016) Mechanisms Underlying Induction of Tolerance to Foods. Immunol Allergy Clin North Am 36: 87-102.
-
Emma Merike Savilahti (2010) Cow's milk allergy and the development of tolerance. Helsinki University Printing House, Helsinki 12-33.
-
Kim KS, Surh CD (2015) Induction of Immune Tolerance to Dietary Antigens. Adv Exp Med Biol 850: 93-118.
-
Commins SP (2015) Mechanisms of Oral Tolerance. Pediatr Clin North Am 62: 1523-1529.
-
Scurlock AM, Vickery BP, Hourihane JO, Burks AW (2010) Pediatric food allergy and mucosal tolerance. Mucosal Immunol 3: 345-354.
-
Carrard A, Rizzuti D, Sokollik C (2015) Update on food allergy. Allergy 70: 1511-1520.
-
Vandenplas Y, Marchand J, Meyns L (2015) Symptoms, Diagnosis, and Treatment of Cow's Milk Allergy. Curr Pediatr Rev 11: 293-297.
-
Netting M, Makrides M, Gold M, Quinn P, Penttila I (2013) Heated allergens and induction of tolerance in food allergic children. Nutrients 5: 2028-2046.
-
Vitaliti G, Cimino C, Coco A, Praticò AD, Lionetti E (2012) The immunopathogenesis of cow's milk protein allergy (CMPA). Ital J Pediatr 38: 35.
-
Nowak-Wezgrzyn A (2015) What makes children outgrow food allergy? Clin Exp Allergy 45: 1618-1620.
-
Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA (2009) Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol 123: 43-52.
-
Yamashita H, Takahashi K, Tanaka H et al. (2012) Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy 67: 201-209.
-
Dang TD, Allen KJ, et al. (2016) Food-allergic infants have impaired regulatory T-cell responses following in vivo allergen exposure. Pediatr Allergy Immunol 27: 35-43.
-
Jing Yang, Fazheng Ren, Hao Zhang et al. (2015) Induction of Regulatory Dendritic Cells by Lactobacillus paracasei L9 Prevents Allergic Sensitization to Bovine β-Lactoglobulin in Mice. J Microbiol Biotechnol 25: 1687-1696.
-
Pastor-Vargas C, Maroto AS, Diaz-Perales A et al. (2015) Sensitive detection of major food allergens in breast milk: first gateway for allergenic contact during breastfeeding. Allergy 70: 1024-1027.
-
Christine C. Johnson, Dennis R. Ownby (2016) Allergies and Asthma: Do Atopic Disorders Result from Inadequate Immune Homeostasis arising from Infant Gut Dysbiosis? Expert Review of Clinical Immunology 12: 379-388.
-
Hoyt AE, Medico T, Commins SP (2015) Breast Milk and Food Allergy: Connections and Current Recommendations. Pediatr Clin North Am 62: 1493-1507.
-
Van Elten TM, van Rossem L, Wijga AH et al. (2015) Breast milk fatty acid composition has a long-term effect on the risk of asthma, eczema, and sensitization. Allergy 70: 1468-1476.
-
Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, et al. (2015) Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 70: 1091-1102.
-
Mosconi E., Rekima A., Seitz-Polski B. et al. (2010) Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 3: 461-474.
-
Kiewiet MB, Gros M, van Neerven RJ, Faas MM, de Vos P (2015) Immunomodulating properties of protein hydrolysates for application in cow's milk allergy. Pediatr Allergy Immunol 26: 206-217.
-
Amanda M. Foiles, Elizabeth H. Kerling, Jo A. Wick et al. (2016) Formula with long-chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr Allergy Immunol 27: 156-161.
-
Jessica Metcalfe, Susan L. Prescott, Debra J. Palmer (2013) Randomized controlled trials investigating the role of allergen exposure in food allergy: where are we now? Curr Opin Allergy Clin Immunol 13: 296-305.
-
Young MC (2015) Taking the leap earlier: the timing of tolerance. Curr Opin Pediatr 27: 736-740.
-
Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, et al. (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372: 803-813.
-
Melissa J. Berry, Jennifer Adams, Helena Voutilainen et al. (2015) Impact of elimination diets on growth and nutritional status in children with multiple food allergies. Pediatr Allergy Immunol 26: 133-138.
-
Vandenplas Y, Abuabat A, Al-Hammadi S, Aly GS, Miqdady MS, et al. (2014) Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow's Milk Protein Allergy. Pediatr Gastroenterol Hepatol Nutr 17: 61-73.
-
Tunis MC, Dawod B, Carson KR, et al. (2015) Toll-like receptor 2 activators modulate oral tolerance in mice. Clin Exp Allergy 45: 1690-1702.
-
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, et al. (2015) International consensus on allergy immunotherapy. J Allergy Clin Immunol 136: 556-568.
-
Järvinen KM, Westfall J, De Jesus M, Mantis NJ, et al. (2015) Role of Maternal Dietary Peanut Exposure in Development of Food Allergy and Oral Tolerance. PLoS One 10: e0143855.
-
Miles EA, Calder PC (2015) Maternal diet and its influence on the development of allergic disease. Clinical & Experimental Allergy 45: 63-74.
-
West CE (2016) Probiotics for allergy prevention. Benef Microbes 7: 171-179.