Trauma Cases and Reviews
Association between Sequential Organ Failure Assessment Score and In-hospital Deaths of Surgical, Critically Ill Patients with Sepsis
Noboru Harada1*, Ken Shirabe2, Shinji Itoh2, Hideaki Uchiyama2, Motoyuki Yamagata3, Fumiaki Kishihara4, Takashi Maeda1, Nao Kinjo5, Kensaku Sanefuji6, Yosuke Kuroda7, Kazutoyo Morita8, Tomoharu Yoshizumi2, Toru Ikegami2, Yo-ichi Yamashita2, Yoshihiko Maehara2 and Study group of refractory disease in hepato-pancreato-biliary surgery
1Department of Surgery, Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital, Hiroshima, Japan
2Department of Surgery and Medical Science, Kyushu University, Fukuoka, Japan
3Department of Surgery, Saiseikai Karatsu Hospital, Saga, Japan
4Department of Surgery, Nakatsu Municipal Hospital, Oita, Japan
5National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
6Oita Red Cross Hospital, Oita, Japan
7Matsuyama Red Cross Hospital, Ehime, Japan
8Fukuoka City Hospital, Fukuoka, Japan
*Corresponding author: Noboru Harada, MD, PhD, Department of Surgery, Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital, 1-9-6 Senda-machi Naka-ku, Hiroshima, 730-8619, Japan, Tel: +81-82-2413111, Fax: +81-82-2460676, E-mail: noharada14@gmail.com
Trauma Cases Rev, TCR-2-026, (Volume 2, Issue 1), Original Article; ISSN: 2469-5777
Received: December 04, 2015 | Accepted: January 11, 2016 | Published: January 13, 2016
Citation: Harada N, Shirabe K, Itoh S, Uchiyama H, Yamagata M, et al. (2016) Association between Sequential Organ Failure Assessment Score and In-hospital Deaths of Surgical, Critically Ill Patients with Sepsis. Trauma Cases Rev 2:026. 10.23937/2469-5777/1510026
Copyright: © 2016 Harada N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: This study was designed to identify factors prognostic of in-hospital deaths of surgical, critically ill patients with sepsis and to evaluate the effects of treatments for sepsis on in-hospital deaths.
Methods: This retrospective cohort study involved 103 patients with sepsis who were hospitalized in the surgical intensive care units of 20 hospitals. Clinical, microbiologic, and laboratory factors, as well as treatments, were compared between patients who survived hospitalization and those who died in-hospital.
Results: The in-hospital mortality and septic shock rates were 24.3% and 19.4%, respectively. Multivariate logistic regression analysis showed that Sequential Organ Assessment (SOFA) score was the only independent predictor of in-hospital death (P = 0.027). Receiver operating characteristic curve analysis of in-hospital death showed that the optimal SOFA score cutoff at admission to the surgical intensive care unit was 8, with in-hospital death rate being significantly higher in the 22 patients with SOFA score > 8 than in the 81 patients with SOFA score ≤ 8 (P = 0.0039). Cox regression analyses by inverse probability treatment weighting to control for selection bias showed that in-hospital death rates were not significantly altered by treatment with intravenous immunoglobulins, renal replacement therapy, or endotoxin-absorbing therapy using polymyxin B.
Conclusions: SOFA score may be prognostic of in-hospital deaths of surgical critically ill patients with sepsis. SOFA score > 8 was associated with a significantly higher in-hospital death rate and should be regarded as a cut-off for intensive treatment in surgical patients with sepsis.
Keywords
SOFA, Septic shock, Surgical critically ill, Prognostic factors
Introduction
Sepsis, defined as infection-induced systemic inflammatory response syndrome (SIRS), is the leading cause of death in non-cardiac critically ill patients [1]. In the United States, nearly 200,000 deaths per year are attributed to sepsis [2]. Worldwide, as many as 20 million people may experience sepsis annually, with a mortality rate of about 35% [3]. Sepsis involves multiple mechanisms, including the release of cytokines and the activation of the complement, coagulation and fibrinolytic systems [4].
The first internationally accepted guidelines to improve outcomes in patients with severe sepsis and septic shock were adopted in 2004 [5,6] and updated in 2008 [7] and 2012 [8,9]. Current guidelines recommend a specific anatomical diagnosis of infection as rapidly as possible, with intervention for source control started within the first 12 hours after diagnosis, if feasible [8,9].
Few studies to date have described the treatment of surgical, critically ill patients with sepsis [10-12]. The results of treatments of sepsis in surgical patients requiring other types of treatment, including burn care, catheter drainage of the source of infection and abdominal surgery under general anesthesia, remain unclear. It is therefore of interest to clarify factors prognostic of survival in surgical, critically ill patients with sepsis.
Moreover, although adjunctive therapies, including intravenous immunoglobulins (IVIG) [13,14], renal replacement therapy [15,16], and endotoxin-absorbing therapy using polymyxin B (PMX) [17], have been reported to reduce the risks of death in septic patients, these treatments have not been universally accepted. Inverse probability of treatment weighting (IPTW) was therefore used to balance the underlying distributive covariates among patients who did and did not receive each adjunctive therapy. IPTW weights the samples using propensity score to reduce the confounding that frequently occurs in cohort studies of the effects of treatment on outcome, and enables estimation of marginal or population-average treatment effects [18].
This study was therefore designed to identify factors prognostic of in-hospital deaths in surgical, critically ill patients with sepsis and to evaluate the effects of treatment of sepsis on patient survival.
Materials and Methods
This retrospective cohort study involved 110 patients who were hospitalized with sepsis in the surgical intensive care units of 20 hospitals affiliated with the Department of Surgery and Medical Science, Graduate School of Medical Sciences, Kyushu University, between January 2012 and December 2013 (Supplemental Table 1). Patients lacking adequate clinical data were excluded.
![]()
Supplemental Table 1: Hospitals included in this Study.
View Supplemental Table 1
Sepsis was defined as an infection with SIRS, defined as the occurrence of at least two of the following criteria: (1) body temperature > 38°C or < 36°C, (2) heart rate > 90 beats per minute, (3) respiratory rate > 20 breaths a minute or PaCO2 < 32 mmHg, and (4) white blood cell count > 12,000/ mm3 or < 4000/mm3 or < 10 % immature forms [4]. Blood samples were drawn when patients first fulfilled the criteria for SIRS. Septic shock was defined as sepsis-induced hypotension, consisting of systolic blood pressure below 90 mmHg, which persisted despite adequate fluid resuscitation [8]. Ileus was defined as any impairment, arrest, or reversal of the normal flow of intestinal contents toward the anal canal.
All patients diagnosed with sepsis and admitted to the surgical intensive care unit before and after surgery were enrolled in this study. Demographic and clinical data retrieved from their medical records included sex, age, underlying disease, location of the primary infection, bacterial species, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Assessment (SOFA) score, blood pressure, heart rate, hematocrit, white blood cell (WBC) count, platelet count, C-reactive protein (CRP), total bilirubin, serum creatinine, fibrinogen, prothrombin activity, fibrinogen degradation products (FDP), D-dimer, and type of surgical intervention for sepsis, including removal or drainage of sites of infection.
Treatment of sepsis, including surgical intervention and antimicrobial therapy, was initiated as soon as possible according to the Surviving Sepsis Campaign guidelines [8,9]. If primary antibiotics were not effective, as determined by WBC count, CRP concentration, and clinical signs such as high fever, IVIG (5 g/kg body weight) was administered for 3 days along with antibiotics [13,14]. Patients with septic shock who experienced acute renal failure, defined as anuria and/or serum creatinine concentration > 4.0 mg/dl, were administered renal replacement therapy, including continuous hemodiafiltration (CHDF) or intermittent hemodialysis, to protect renal function and remove inflammatory cytokines [15,16]. If hemodynamic stability could not be restored by adequate fluid resuscitation and vasopressor therapy, patients were administered PMX [16]. In addition, mechanical ventilation was administered to patients with sepsis-induced acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Fluid resuscitation and catecholamine were administered to maintain circulation in patients with septic shock, thus preventing the development of a more critical condition that could result in multiple organ failure (MOF) and death [8,9].
Statistical Analysis
For continuous variables, nonparametric analyses were performed using Wilcoxon rank-sum tests. Categorical variables were compared using chi-squared or Fisher's exact tests. The Kaplan-Meier method was used to construct cumulative survival curves. To identify factors independently predictive of in-hospital death, the factors found to be significant on univariate analysis were assessed by multivariate logistic regression analyses. The SOFA score cut-off value for in-hospital death was calculated using the receiver operator characteristic (ROC) curve method [19].
IPTW analysis was performed to overcome possible biases from differences in distribution among patients who did and did not receive each treatment [18,20]. Propensity scores by IPTW were calculated using a Cox regression model to predict the probability of each patient receiving each treatment on the basis of eight clinical variables: age; sex; APACHE II score; SOFA score; positive (vs. negative) blood culture; and treatment including IVIG, renal replacement therapy, and PMX. Following balancing by IPTW, the between group differences in in-hospital death rates were evaluated by the log-rank test. Statistical analyses were performed using the R statistical programming environment (the Comprehensive R Archive Network, http://cran.md.tsukuba.ac.jp) and JMP 9.0 software (SAS Institute, Cary, NC). Four basic R programming packages were used for IPTW analyses: Rcmdr, survival, RcmdrPlugin, survival, and Epi. Statistical significance was defined as a P value < 0.05.
Results
Of the 110 patients enrolled initially, seven were excluded owing to uncertainties about initial recognition of SIRS. The primary causes of sepsis in the 103 analyzed patients included acute peritonitis (n = 50), acute cholecystitis (n = 17), acute cholangitis (n = 9), ileus (n = 7), gangrene of the lower limbs (n = 4), liver abscess (n = 2), postoperative catheter infection (n = 2), postoperative acute enteritis (n =2), postoperative pneumonitis (n = 2), postoperative urinary tract infection (n = 2), postoperative infectious endocarditis (n = 1), extensive burn (n = 1), abdominal compartment syndrome (n = 1), esophageal perforation (n = 1), postoperative leakage of esophageal anastomosis (n = 1), and postoperative pancreas fistula (n = 1). Table 1 shows the relationships between the primary cause of sepsis and surgical treatment in these patients. Of the 103 patients, 60 (58.3%) underwent surgery under general anesthesia, and 24 (23.3%) underwent surgical drainage under local anesthesia. Adjunctive treatments included IVIG therapy in 33 patients (32.0%), renal replacement therapy in 18 (17.5%), and PMX in 21 (20.4%).
![]()
Table 1: Causes of sepsis and types of treatment in surgical critically ill patients with sepsis.
View Table 1
The mean age of the 103 patients (65 men and 38 women) was 72.3 ± 1.2 years (range, 37-99 years). Twenty-five (24.3 %) patients died in-hospital and 20 (19.4 %) experienced septic shock. The mean hospital stay was 36.7 ± 2.5 days (range, 2-100 days). The mean APACHE II and SOFA scores at admission were 15.5 ± 0.8 and 5.9 ± 0.4, respectively.
Univariate analysis identified four variables as risk factors for in-hospital deaths in these patients: heart failure as a comorbidity (P = 0.036), APACHE II score (P = 0.002), SOFA score (P < 0.0001), and prothrombin activity (P = 0.006). Of the 78 in-hospital survivors, nine had chronic renal failure, including two on hemodialysis, with no differences in rates of chronic renal failure between survivors and non-survivors (P = 0.109). Multivariate logistic regression analysis showed that SOFA score was the only independent predictor of in-hospital death (P = 0.027, odds ratio 1.24; 95% confidence interval 1.03-1.52, Table 2 and Table 3).
![]()
Table 2:Univariate analysis of risk factors for in-hospital deaths among surgical, critically ill patients with sepsis Results reported as mean ± standard error.
View Table 2
![]()
Table 3: Multivariate analysis of risk factors for in-hospital deaths among surgical, critically ill patients with sepsis.
View Table 3
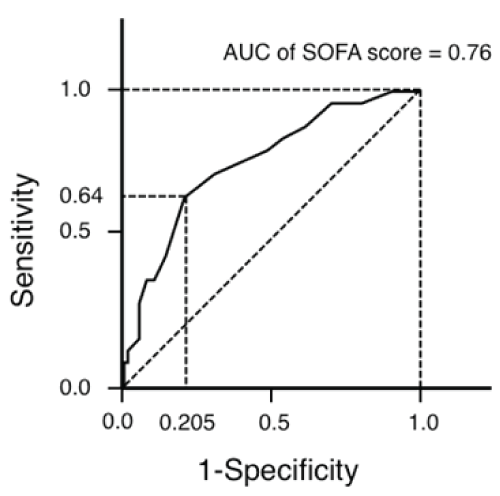
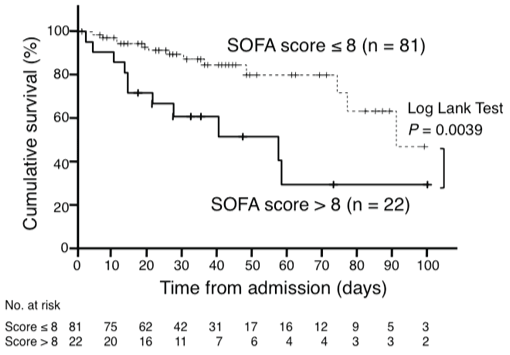
ROC curve analysis and determination of the area under the ROC curve (AUC) showed that SOFA score was effective in distinguishing surgical, critically ill patients with sepsis who did and did not survive in-hospital. The AUC of SOFA score was 0.76. ROC curve analysis for in-hospital deaths showed that the optimal SOFA score cutoff at admission to the surgical intensive care unit was 8 (sensitivity; 0.64, 1-specificity; 0.25, Figure 1). The in-hospital death rate was significantly higher in the 22 patients with SOFA score > 8 than in the 81 patients with SOFA score ≤ 8 (P = 0.0039, Figure 2).
.
Figure 1: Receiver operating characteristic (ROC) curve analysis of SOFA score distinguishing in-hospital survivors and non-survivors among surgical, critically ill patients with sepsis. The area under the ROC curve (AUC) was 0.76.
View Figure 1
.
Figure 2: Kaplan-Meier survival curves of surgical, critically ill patients with sepsis having SOFA scores ≤ 8 and > 8. The 22 patients with SOFA score > 8 had a significantly higher in-hospital death rate than the 81 patients with SOFA score ≤ 8 (P = 0.0039).
View Figure 2
The microorganisms isolated from these patients are shown in table 4. Gram-positive microorganisms were cultured from 37.9 % of these patients; Gram-negative organisms from 34.0 %; and Candida species from 2.9%. In-hospital deaths were not associated with either Gram-positive or Gram-negative organisms. Escherichia coli was associated with the main source of infection in patients with septic shock (P = 0.021), but not with in-hospital deaths. None of the other microorganisms was associated with either septic shock or in-hospital death rate. Antimicrobial treatment was administered to all patients. Only six patients (7.8%) received inadequate antimicrobial treatment, but this was not related to in-hospital mortality (P = 0.620). Of these six patients, three were infected with methicillin-resistant Staphylococcus aureus (MRSA), two with Candida glabrata, and one with Enterococcus faecium; four (67%) of these patients received empirical treatment with carbapenems.
![]()
Table 4: Microorganisms associated with in-hospital deaths among surgical, critically ill patients with sepsis.
View Table 4
We also assessed the effects of adjunctive treatment with IVIG, renal replacement therapy, and PMX in patients with sepsis. Following IPTW using eight variables: age, sex, positive blood culture, APACHE score, SOFA score, and treatment with IVIG, renal replacement therapy, and PMX, we found that the weighted in-hospital death rates were similar in patients who did and did not receive each treatment (Table 5).
![]()
Table 5: Analyses of the effects of adjunctive treatments of surgical, critically ill patients with sepsis by inverse probability of treatment weighting.
View Table 5
Discussion
Sepsis is the leading cause of death of surgical patients pre- and postoperatively [21-23]. Once the sources of infection are identified, surgical intervention plus antibiotics are recommended as treatment [8,9]. However, the in-hospital death rate remains high, even following these guidelines [8,9]. The mortality rate in surgical patients with sepsis was reported to be 25.5%, similar to the rate in our study (24.3%) [22]. However, the effectiveness of adjunctive treatments, such as IVIG, renal replacement therapy, and PMX, remains unclear. Moreover, few reports have assessed surgical treatments associated with sepsis, risk factors associated with mortality of surgical, critically ill patients, and microbes associated with sepsis [23]. Although epidemiology and outcomes before and after admission to the surgical intensive care unit have been analyzed [10,11], prognostic factors and treatment results in septic surgical patients remain unclear. The duration of SIRS has been reported to be significantly prognostic of sepsis in medical and surgical units (P = 0.015) [22]. Our study showed that SOFA score may be potentially prognostic of in-hospital deaths in surgical, critically ill patients with sepsis. These results are in accordance with a study that found an association between SOFA score and outcomes in patients admitted to the intensive care unit [24]. Serial measurements of SOFA score during the first week were very useful in predicting the outcome of sepsis, whereas APACHE II scores on the day of admission were not reliable in predicting mortality [25].
SOFA score is simple and objective, allowing the calculation of the number of dysfunctional organ systems and their severity among six organ systems (respiratory, coagulatory, liver, cardiovascular, renal, and neurologic) [26]. Moreover, SOFA score can measure dysfunction of individual and aggregate organs [27]. SOFA score has been used to evaluate patients with multiple organ dysfunction syndromes (MODS) and their survival [26]. Organ failure may worsen outcomes in surgical critically ill patients with sepsis. Indeed, we found that in-hospital death rates were significantly higher in patients with SOFA scores > 8 than ≤ 8 at admission. SOFA score at admission may therefore suggest the type of primary treatment, including adjunctive therapy. In surgical patients, organ dysfunction at sites other than that of surgery may be more strongly associated with death than transient vital signs and serum chemistry included in the APACHE II score on admission [28].
Although our univariate analysis found that APACHE II score was prognostic of in-hospital deaths, this finding was not observed on from multivariate analyses. APACHE II score is used in intensive care units to assess disease severity. However, SOFA score may be superior to APACHE II score in predicting in-hospital deaths of surgical, critically ill patients with sepsis, because SOFA score, which includes platelet count, may objectively evaluate failure of individual and aggregate organs [26]. Coagulopathy, such as low platelet count, has been associated with sepsis-induced disseminated intravascular coagulation (DIC) [29] and may lead to a severe disease condition or in-hospital death. A prospective survey in Japan also demonstrated that SOFA and DIC scores were consistently higher in nonsurvivors than survivors on the day of admission and 3 days later [23].
Microbial assessment found that E. coli infection was frequently associated with septic shock, including in patients with community-acquired bloodstream infection [30]. Because none of the other microorganisms assayed was associated with septic shock or in-hospital death and only 7.8% of patients received inadequate antimicrobial treatment, surgical, critically ill patients with sepsis should be treated with a broad empirical antimicrobial agent such as a carbapenem. Infections with MRSA and Candida species are of particular concern. The frequency of invasive fungal infections in developed countries has increased because of advances in medical management [31]. Although invasive Candidiasis is frequently intraabdominal in critically ill surgical patients, prompt antifungal therapy and adequate source control may yield good outcomes [32]. Resistance to azoles, particularly fluconazole, should be considered when starting an empirical treatment [32].
Although we attempted to assess the effects of adjunctive treatment, including IVIG, renal replacement therapy, and PMX, in patients with sepsis by IPTW analysis, we found that none had a significantly positive effect on this patient population. However, our study had several limitations. First, relatively few patients received each type of adjunctive treatment. Second, this was a retrospective cohort study assessing the causes of sepsis in Japanese patients. Thus, the results were not externally validated and were limited to patients of Japanese ethnicity. Third, we could not plan a prospective study to clarify the effectiveness of each adjunctive therapy in surgical critically ill patients with sepsis. However, well-designed randomized controlled studies and/or meta-analyses may result in external validity, as well as assessing these adjunctive treatments in patients of varying ethnicity.
In conclusion, this study found that SOFA score may be prognostic of in-hospital deaths of surgical, critically ill patients with sepsis. SOFA > 8 was associated with significantly higher in-hospital death rate and may be a cut-off for the necessity of intensive treatment in these patients.
Conflict of Interest
All authors declare no conflicts of interest.
Compliance with Ethical Requirements
The study protocol was approved by the ethics committees of all participating hospitals, and study information was disclosed to the patients or their survivors by members of the Department of Surgery and Medical Science, Graduate School of Medical Sciences, Kyushu University.
Acknowledgments
The authors thank Dr. Y. Fujinaka (Matsuyama Red Cross Hospital), Dr. R. Nakanishi (Matsuyama Red Cross Hospital), Dr. T. Iso (Social Insurance Nakabaru Hospital), Dr. Y Ikeda (National Hospital Organization Kyushu Cancer Center), Dr. T, Rikimaru (Munakata Medical Association Hospital), Dr. K. Nomoto (Saiseikai Yahata General Hospital), Dr. H. Hasegawa (Saiseikai Yahata General Hospital), Dr. S. Maehara (Matsuyama Red Cross Hospital), Dr. E. Adachi (Oita Prefectural Hospital), Dr. K. Tada (Oita Prefectural Hospital), Dr. M. Noda (Oita Prefectural Hospital), Dr. K. Umeda (Oita Prefectural Hospital), Dr. K. Yamamoto (Imari Arita Kyoritsu Hospital), Dr. H. Sonoda (Imari Arita Kyoritsu Hospital), Dr. G. Anegawa (Imari Arita Kyoritsu Hospital), Dr. T. Nishizaki (Matsuyama Red Cross Hospital), Dr. Y. Soejima (Matsuyama Red Cross Hospital), Dr. T. Honbo (Matsuyama Red Cross Hospital), Dr. K. Iwaki (Oita Red Cross Hospital), Dr. S. Kai (Oita Red Cross Hospital), Dr. K. Fukuzawa (Oita Red Cross Hospital), Dr. T. Ezaki (Fukuoka Higashi Medical Center), Dr. T. Ohga (Fukuoka Higashi Medical Center), Dr. H. Uehara (Nakatsu Municipal Hospital), Dr. T. Okada (Nakatsu Municipal Hospital), and Dr. Y. Nakaji (Saiseikai Karatu Hospital) for helping with data collection.
References
-
Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, et al. (1995) The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273: 117-123.
-
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303-1310.
-
Daniels R (2011) Surviving the first hours in sepsis: getting the basics right (an intensivist's perspective). J Antimicrob Chemother 66 Suppl 2: ii11-23.
-
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250-1256.
-
Dellinger RP, Carlet JM, Ramsay G, Zimmerman JL, Vincent JL, et al. (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32: 858-873.
-
Dellinger RP, Carlet JM, Ramsay G, Zimmerman JL, Vincent JL, et al. (2014) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med 30: 536-555.
-
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, et al. (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34: 17-60.
-
Dellinger RP, Levy MM, Webb SA, Beale RJ, Vincent JL, et al. (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41(2): 580-637.
-
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165-228.
-
Rhodes A, Moreno RP, Metnitz B, Hochrieser H, Bauer P, et al. (2011) Epidemiology and outcome following post-surgical admission to critical care. Intensive Care Med 37: 1466-1472.
-
Elias AC, Matsuo T, Grion CM, Cardoso LT, Verri PH (2012) Incidence and risk factors for sepsis in surgical patients: a cohort study. J Crit Care 27: 159-166.
-
De Waele J, De Bus L (2014) How to treat infections in a surgical intensive care unit. BMC Infect Dis 14: 193.
-
Alejandria MM, Lansang MA, Dans LF, Mantaring JB (2002) Intravenous immunoglobulin for treating sepsis and sepotic shock. Cochrane Database Syst Rev CD001090.
-
Turgeon AF, Hutton B, Fergusson DA, McIntyre L, Tinmouth AA, et al. (2007) Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 146: 193-203.
-
Mehta RL, McDonald B, Gabbai FB, Pascual MT, Farkas A, et al. (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60: 1154-1163.
-
Ronco C, Bellomo R, Homel P, Dan M, Piccinni P, et al. (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356: 26-30.
-
Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, et al. (2007) Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 11: R47.
-
Austin PC (2013) The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32: 2837-2849.
-
Lusted LB (1971) Signal detectability and medical decision-making. Science 171: 1217-1219.
-
Harada N, Shirabe K, Maeda T, Kayashima H, Ishida T, et al. (2015) Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J Surg 39: 1044-1051.
-
Doklestic SK, Bajec DD, Djukic RV, Bumbasirevic V, Detanac AD, et al. (2014) Secondary peritonitis - evaluation of 204 cases and literature review. J Med Life 7: 132-138.
-
Sugita H, Kinoshita Y, Baba H (2012) The duration of SIRS before organ failure is a significant prognostic factor of sepsis. Int J Emerg Med 5: 44.
-
Ogura H, Gando S, Saitoh D, Takeyama N, Yamashita N, et al. (2014) Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother 20: 157-162.
-
Routsi C, Pratikaki M, Sotiropoulou C, Platsouka E, Markaki V, et al. (2007) Application of the sequential organ failure assessment (SOFA) score to bacteremic ICU patients. Infection 35: 240-244.
-
Desai S, Lakhani JD (2013) Utility of SOFA and APACHE II score in sepsis in rural set up MICU. J Assoc Physicians India 61: 608-611.
-
Vincent JL, Moreno R, Takala J, Reinhart CK, Suter PM, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707-710.
-
Artero A, Zaragoza R, Camarena JJ, Sancho S, Gonzalez R, et al. (2010) Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care 25: 276-281.
-
Vincent JL, Nelson DR, Williams MD (2011) Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med 39: 1050-1055.
-
Gando S, Saitoh D, Ishikura H, Mashiko K, Tsubota T, et al. (2013) A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care 7: R297
-
de Ruiter J, Weel J, Manusama E, Kingma WP, van der Voort PH (2009) The epidemiology of intra-abdominal flora in critically ill patients with secondary and tertiary abdominal sepsis. Infection 37: 522-527.
-
Aki K, Okubo Y, Nanjo H, Ishiwatari T, Masuda H, et al. (2015) Genomic Analysis of Single Nucleotide Polymorphisms Asp299Gly and Thr399Ile in Japanese Patients with Invasive Aspergillosis. Jpn J Infect Dis 68: 330-332.
-
Aguilar G, Delgado C, Corrales I, Izquierdo A, Gracia E, et al. (2015) Epidemiology of invasive candidiasis in a surgical intensive care unit: an observational study. BMC Res Notes 8: 491.





