Journal of Rheumatic Diseases and Treatment
TB: Falsely Accused? (A Case Report and Discussion)
Kelsy Greenwald* and Jo Ann Ball
Rheumatology, Desert Medical Advances, USA
*Corresponding author: Kelsy Greenwald, Rheumatology, Desert Medical Advances, 72855 Fred Waring Dr., Palm Desert, CA 92260, USA, E-mail: bdelph@dc.rr.com
J Rheum Dis Treat, JRDT-2-042, (Volume 2, Issue 4), Case Report; ISSN: 2469-5726
Received: August 13, 2016 | Accepted: September 24, 2016 | Published: October 03, 2016
Citation: Greenwald K, Ball JA (2016) TB: Falsely Accused? (A Case Report and Discussion). J Rheum Dis Treat 2:042. 10.23937/2469-5726/1510042
Copyright: © 2016 Greenwald K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Interferon-Gamma Release Assays (IGRAs) in the past decade have become ubiquitous screening for tuberculosis (TB). Development of these interferon release tests helped screen for TB in patients at high risk, specifically those with human immunodeficiency virus (HIV). The blood test was designed to strongly stimulate interferon release when blood was exposed to a tube laced with TB antigens. Cut points were established so that any interferon measurement over 35 IU is considered a "positive" test for TB.
Sero-positive rheumatoid patients (RA) spontaneously produce interferon. During an IGRA test in a sero-positive RA patient, the interferon levels are often over 35 IU, but without exposure to TB. We found the interferon test results from 16 sero-positive RA patients correlated with the level of cyclic citrullinated protein (p < 0.02). After treatment of RA, the IGRA test Interferon-Gold, alternated between positive and negative. This test designed originally for HIV patients, is not ideal for sero-positive RA patients where interferon is produced spontaneously.
This report describes a small series of 16 sero-positive RA patients without TB in the United States, all with false positive Interferon-Gold testing. The interferon test is a screening test only, and not a diagnostic test. As with many diagnoses in medicine, tests should be evaluated in context. In one illustrative case, the Interferon-Gold test was misleading, incorrect treatment administered, and the RA patient died. TB may be falsely accused. TB is not diagnosed by an interferon level in RA, and should be diagnosed by culture, m-RNA, or acid-fast positive smear. In RA, the better TB screening test is the skin purified protein derivative (PPD) which tests type 4 delayed hypersensitivity and not interferon levels. Data has shown with use of biologic and immune modulating therapies, that the leading life-threatening infections are bacterial pneumonias. False positive TB interferon tests are not helpful.
Keywords
Rheumatoid arthritis, Tuberculosis, Cyclic citrullinated peptide, Interferon tuberculosis test, Latent tuberculosis
There is concern about the risk of developing infection with tuberculosis (TB) in patients taking tumor necrosis factor inhibitors [1]. Evaluation for TB before initiating therapy with tumor necrosis factors is clearly stated in the package insert for each tumor necrosis factor available. The best evaluation for risk of tuberculosis requires both context of background prevalence and clinical symptoms. Here we discuss errors in evaluating TB using screening interferon tests; incubating cells from patients with autoimmune disease can result in high levels of interferon and result in values very different from other populations [2]. Interferon testing for TB in a test tube might be substituted for diagnosis inappropriately.
The following case report illustrates some of the problems in a clinical setting for the diagnosis of TB. There is a misdiagnosis caused by a lab test. Following the case report follows a series of other rheumatoid arthritis (RA) patients who were falsely identified as exposed to TB.
In the first illustrative case, a 71-year-old female with sero-positive RA on methotrexate, had adalimumab added to her treatment regimen. Her TB evaluation included a negative chest radiograph, negative history of TB exposure, and a negative interferon TB test (QuantiFERON-Gold, QFT-G).
A year later, she developed productive cough, fever, and dyspnea with radiographic infiltrates. The repeat QFT-G test was positive, and treatment began with INH, rifampicin, and ethambutol. A few days later, the patient deteriorated and died [3].
Subsequent tests showed no acid fast bacillus on sputum smear, sputa submitted for both RNA analysis and culture was negative for TB. The patient died of bacterial sepsis.
The TB test tube interferon result was clearly misinterpreted as a diagnostic test. It is a screening test only, and at our center we discovered 16 false positive QFT-G TB tests in 2011. Screening for latent TB by QFT-G testing was performed on 126 sero-positive RA patients and 16 of these were adjudicated as false positive (13%). No patient had cough, fever, no travel outside the area, and no TB exposure. Each had a negative TB skin test (PPD) and a clear chest X-ray. The area is rural desert with 10% humidity and no mass public transportation. The incidence of TB in the desert region is 0.0007/100 patient years. Infectious disease experts concluded these were false QFT-G results. All but one patient had very high positive CCP serology and the QFT-G result correlated with the level of CCP (p < 0.02, Table 1). None of the patients were treated for latent TB, subsequent biologic therapy was instituted with careful observation and no signs or symptoms of TB occurred after three years. Nasopharyngeal fluid submitted for RNA analysis has been negative for TB annually. Repeat QFT-G testing has been alternately negative, then positive, then negative and not consistent in any of the 16 patients over 3 years.
![]()
Table 1: RA baseline characteristics.
View Table 1
According to Bayes Theorem [4] (standard for evaluation of tests), the probability of disease (incidence) must be higher than the error of the lab test. In our institution, the incidence of TB, 0.0007/100 patient years, is far smaller than the error in the QFT-G test, therefore, a poor screening test in our area. Multiple published reviews [5] and MedDRA [6] reports on approved biologics in clinical trials, have found the vast majority of TB in Asia and Eastern Europe, with few cases in North America. The incidence of TB has been steadily declining in the USA; the national rate in US born citizens is about 1 per 100,000 [7].
Reasons for a false positive QFT-G test:
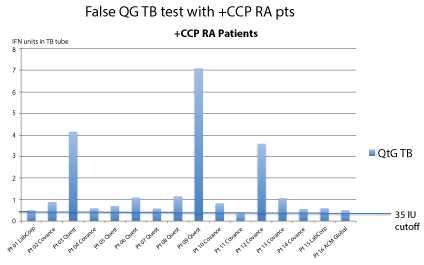
1. The test measures interferon after prolonged incubation; interferon is spontaneously produced by RA patients. The "false positive" tests from QFT-G are generally borderline low positive (Figure 1).
2. The QFT-G serum test is complicated, involving three tubes per patient, incubated the same day as the specimen is drawn ("incubate as soon as possible") [8]. Covance, Quest, and Quintiles have sent our site incorrect information; a) labs instructed to send tubes without incubation first, b) another sent the wrong three tubes, and c) several times the lab technician recorded the positive "mitogen" tube results into the wrong column.
3. The QFT-G test can be invalid after a skin PPD test [9]. The TB skin test boosts the interferon response yet QFT-G tests do not ask about a prior PPD. This results in false positive QFT-G testing where PPD is in routine use (hospitals, schools, prisons, etc) [10].
4. QFT-G testing was developed in the 1990's to assess TB in immune-compromised HIV patients at high risk with low CD4 counts. In contrast, RA patients often have high baseline interferon with incubation. We found a statistical correlation of interferon level in the TB tube with the level of CCP (p < 0.02). The increase in interferon activity increased with BiP (a cyclic citrullinated peptide) is well documented [11]. In RA patients, the QFT-G test may be measuring non-specific auto-immune activity (Table 1).
It is unclear if QFT-G should be used in sero-positive RA patients where auto-immune activity may invalidate the test. When QFT-G testing is performed, immediate processing with results for all three tubes reported is necessary. Prior PPD also may invalidate QFT-G testing. Lastly, per the CDC, "with a test specificity approaching 99%, when the prevalence of M. tuberculosis infection is < 1%, the majority of positive results will be false positives" [9]. It is important to recognize that the most common serious infection with biologic therapy is bacterial infection, not TB [11]. In the fatal initial case presented here, a false positive QFT-G led to incorrect therapy and death. The better test for screening TB in auto-immune populations is the Mantoux skin test which does not involve measurement of interferon.
References
-
Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, et al. (2009) Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Am Rheum Dis 69: 522-528.
-
Shoda H, Hanata N, Sumitomo S, Okamura T, Fujio K, et al. (2016) Immune responses to mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. Sci Rep 6: 22486.
-
Medwatch #FK2015RO000025 (2016) Individual case safety reports from the FAERS database can be obtained by sending a Freedom of Information request to the FDA.
-
Rifkin RD, Hood WB Jr (1977) Bayesian analysis of electrocardiographic exercise stress testing. N Engl J Med 297: 681-686.
-
Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, et al. (2015) Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 10: 1-6.
-
(2016) MedDRA protocol #RA0077, Table 3.1.
-
Centers for Disease Control and Prevention.
-
QuantiFERON-TB Gold Elisa package insert.
-
Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S (2010) Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection 59: 1-25.
-
Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, et al. (2014) Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in health care workers in the United States. Am J Respir Crit Care Med 189: 77.
-
Bodman-Smith MD, Corrigall VM, Kemeny DM, Panayi GS (2003) A putative autoantigen in rheumatoid arthritis, stimulates IL-10-producing CD8-positive T cells from normal individuals. Rheumatology 42: 637-644.