Differences in Dietary Components and Oxidative Stress Markers between Cervical Cancer Patients and Matched Controls
Gabriela Gutierrez-Salmean1, Mayra Acosta2, Fabiola Arellano2, Karolina Alvarez-Altamirano3, Veronica Ruiz-Manon4, Guillermo M Ceballos-Reyes1 and Vanessa Fuchs-Tarlovsky5*
1Laboratorio de Investigación Integral Cardiometabólica. Sección de Estudios de Posgrado e Investigación. Escuela Superior de Medicina, Instituto Politécnico Nacional, México
2Universidad Iberoamericana Ciudad de México, México
3Universidad Autónoma de Nuevo León, México
4Universidad Autónoma del Estado de México, México
5Servicio de Oncología, Hospital General de México, México
*Corresponding author:
Vanessa Fuchs-Tarlovsky, Hospital General de México. Servicio de Oncologia, Pabellón 111, Doctor Balmis 148. Col. Doctores. Del. Cuauhtémoc. 06726 México, D. F., Tel: (52) (55) 2789 2000, E-mail: fuchsvanessa@yahoo.com
J Nutri Med Diet Care,
Volume 1, Issue 1,
JNMDC-1-004,
Review Article
Received: June 17, 2015: Accepted: June 30, 2015: Published: July 03, 2015
Citation: Gutierrez-Salmean G, Acosta M, Arellano F, Alvarez-Altamirano K, Ruiz-Manon V, et al. (2015) Differences in Dietary Components and Oxidative Stress Markers between Cervical Cancer Patients and Matched Controls. J Nutri Med Diet Care 1:004
Copyright: © 2015 Gutierrez-Salmean G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background and objective: Cervical cancer is a leading cause of death among women in developing countries. Diet has been suggested to play an important role in the pathophysiology of malignant tumors as an imbalance between dietary antioxidant intake and free radical production-from the inflammatory state-results in oxidative stress, which may contibute to both initiation and progression of carcinogenesis. The aims of the present study were to assess the difference regarding dietary intake and oxidative stress plasmatic markers differences between cervical cancer patients and matched controls.
Methods: A case-control study -nested within a larger clinical trial- including 100 patients diagnosed with locally advanced cervical cancer and 80 women without the malignant disease was conducted. A food frequency questionnaire was used for assessing diet. Plasmatic oxidative stress markers -malondialdehyde and free carbonyls- were also determined.
Results: Patients with cervical cancer presented a significantly higher body mass index and higher consumption of dietary protein and fat, niacin, cholesterol, sodium, cyanocobalamin, vitamin E, and zinc. A significantly lower intake of vitamin C was found in the case group. MDA levels were found to be statistically higher in cervical cancer patients while free carbonyls did not show significant differences.
Conclusions: A high-fat/high-protein diet, together with lower consuption of ascorbic acid may contribute to an increase risk of developing cervical cancer. However, further prospective and longitudinal research should be promoted in order to elucidate such associations.
Keywords
Antioxidants, Case-control study, Cervical cancer, Diet, Oxidative stress
Introduction
According to the World Health Organization (WHO), cancer is a leading cause of mortality worldwide, accounting for approximately 13% of all deaths [1]. Among women, cervical cancer is the third most common malignancy; its incidence and mortality is significantly higher in developing countries [2].
Infection from Human Papilloma Virus (HPV) is recognized as the most common cause of such gynecologic cancer [3]; however, certain components within lifestyle -e.g., tobacco smoking, excessive alcohol drinking, use of oral contraceptives, and obesity- are also considered as risk factors [4]. Obesity has been implicated in the pathophysiology of several neoplasms, such as breast, colon, prostate, stomach, and cervical cancers. For its side, dietary components with the strongest association to these diseases include excessive energy (caloric) intake, fat, cholesterol, and alcohol, together with an insufficient consumption of fiber, calcium, vitamins A, E and C, folic acid, and selenium [5].
All of the above participate in the redox balance. Whenever such equilibrium is disrupted, oxidative stress develops as a result of either excess free radical production or insufficient antioxidant mechanisms (both endogenous and exogenous) [6]. In turn, this aberrant state has been suggested to be closely linked to cancer, as the latter implies chronic inflammation that may contribute to the transformation of normal cells to tumor cells [7].
Due to all the mentioned variables, we conducted a study aimed to assess whether diet and oxidative stress markers differed between cervical cancer patients and women without the malignancy, and thus could be considered as potential risk factors.
Methods
A case-control study -nested to a larger clinical trial- including patients diagnosed with locally advanced cervical cancer and matched (for age, socioeconomic status, education, etc.) controls without the malignant disease was conducted at Hospital General de México, O.D. from February to December, 2008. Exclusion criteria were obesity (body mass index ≥ 30kg/m2), diabetes, and hypertension.
Prior to any antineoplastic treatment, diet was assessed with a food frequency questionnaire, validated for Mexican population [8]. Further, a 5mL blood sample was taken in order to determine plasmatic malondialdehyde (MDA) and free carbonyls as oxidative stress markers through colorimetric methods (as reported in the literature) [9-11].
The protocol was approved by the Institutional (Hospital General de México. O.D.) Ethics and Research committees. All participants signed a written informed consent before any procedure. The study was conducted in agreement with local law regulation [12], Helsinki Declaration [13], and Good Clinical Practice guidelines [14].
Results are presented as mean ± standard error, unless otherwise stated. Student's independent t-tests were performed and a statistical significance was considered when p-value was <0.05.
Results
The study included 100 cases with diagnosed locally advanced cervical cancer and 80 matched controls without the malignant disease. As described in figure 1, patients with malignant disease showed a significantly higher body mass index (BMI) (27.07 ± 0.52 vs. 23.60 ± 0.19kg/m2) and MDA levels (9.82 ± 0.75 vs. 6.98 ± 0.48). Plasmatic carbonyls were not different between cases and controls; however, a lower value was found in volunteers without cancer (1.50 ± 0.14 vs. 1.17 ± 0.09).
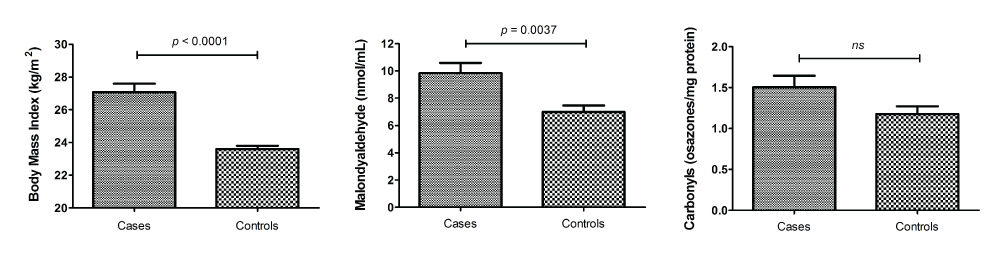
.
Figure 1: Comparison of body mass index, plasma malondialdehyde and carbonyls (as markers of oxidative stress) in cervical cancer patients (cases) and
matching controls. p-values are shown after performing Student’s t-test for independent samples.
View Figure 1
Table 1 shows the data obtained from the analysis of food frequency questionnaire. Even though total daily energy intake was not different between groups, macronutrient distribution had statistically significant differences: while control's diet was balanced-according to national recommendations-, patients with cervical cancer were found to consume a high-protein and high-fat diet, concomitant to a lower intake of carbohydrates.
![]()
Table 1: Data analysis from food frequency questionnaire
View Table 1
Significantly higher intake of niacin, cholesterol, sodium, cyanocobalamin, vitamin E, fats (saturated, monounsaturated, and polyunsaturated), and zinc in the case group was found. In addition, significantly lower amounts of calcium, vitamin C, phosphorous, and water were consumed by cancer patients.
Discussion
Like other malignant diseases, cervical cancer have been proven to enhance oxidative stress both by decreasing antioxidant defense molecules (e.g., glutathione) [15], and by generating reactive oxygen species [16]. In the present study, this fact was confirmed as plasma MDA was significantly higher in the case group; such phenomenon has also been described by other authors [17-19], who suggest that oxidative stress may play an important role in the pathogenesis of cervical malignant disease. However, because of study designs -including the present- it has not been possible to conclude such statement, as these investigations are retrospective hence susceptible to bias.
A novel aspect of the study was the evaluation of nutrition status between cases and controls. Cases presented a significantly higher BMI than controls, in fact, the mean value fell within the overweight criteria range. The importance of such finding is that overweight and obesity are two conditions that have also been associated with oxidative stress since they, as cancer does, enhance the secretion of pro-inflammatory molecules which, in turn, increase reactive species [20].
For its side, an inadequate diet has also been proposed as a potential risk factor for all cancers. Although other studies have not found associations between dietary intake (e.g., fruit, milk, or specific nutrients such as vitamin D or folic acid, etc.) [21,22], similar to the findings in this study, meta-analysis have indicated that women with cervical dysplasia usually have significantly lower intakes of dietary antioxidants-considered as preventive factors for the development of neoplasms-including vitamin C, vitamin A, and carotenes [23,24]. Zinc, for its side, serves as cofactor for antioxidant enzymes (i.e., superoxide dismutase); thus other authors have proposed that zinc deficiency may be a risk factor for cervical cancer [25]. However, cancer patients were found to, unexpectedly, report significantly higher intakes of such mineral. Interestingly, there are few investigations on this regard and, in fact, they zinc is positively associated with gynecological cancer [26], although no clear molecular mechanisms explaining a direct causal relationship have been proposed.
Statistically significant lower dietary calcium vs. the control group was also found among the cases. Evidence regarding this mineral and malignant disease has been previously published: a group of researchers found that higher intakes of calcium were negatively associated with the risk of developing colon and rectal cancer [27]. Such phenomenon has also been described for cervical cancer [28]. Again, no conclusive mechanisms have been established but preclinical studies suggest that calcium is able to modulate cell differentiation, proliferation, adhesion, migration and death [29,30], thus higher intakes may exert a protective effect against gynecologic cancerous lesions.
Finally, the control group presented with normal total energy value distribution from macronutrients, while the cases group had a high-fat, high-protein, low-carbohydrate diet, together with mean values of cholesterol and sodium that exceeded the recommended daily allowances (<300mg and <2400mg, respectively). Moreover, this same group consumed significantly higher amounts of saturated fat. Such type of diet corresponds to that of a Western pattern, which has been associated with tumorigenesis in both preclinical and clinical scenarios [31].
An inadequate diet has been associated with an increased risk for all types of cancer, including cervical. Several dietary and nutrition status components may, in turn, increase the already disrupted redox-balance within the organism. However, there is a lack of scientific literature regarding specific relationships between food take and carcinogenesis. The findings presented in this study, clearly indicate that more research should be promoted in order to improving medical nutrition therapy at the primary and secondary levels of prevention for cervical cancer.
References
-
International Agency of Research in Cancer. Globocan 2008: fast stats.
-
Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, et al. (2010) Cervical cancer prevention: new tools and old barriers. Cancer 116: 2531-2542.
-
Faridi R, Zahra A, Khan K, Idrees M (2011) Oncogenic potential of Human Papillomavirus (HPV) and its relation with cervical cancer. Virol J 8: 269.
-
Bosch FX, Muñoz N, de Sanjosé S, Izarzugaza I, Gili M, et al. (1992) Risk factors for cervical cancer in Colombia and Spain. Int J Cancer 52: 750-758.
-
Khan N, Afaq F, Mukhtar H (2010) Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett 293: 133-143.
-
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8: 1865-1879.
-
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49: 1603-1616.
-
Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, et al. (1998) Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex 40: 133-140.
-
lowry Oh, Rosebrough Nj, Farr Al, Randall Rj (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
-
Erdelmeier I, Gérard-Monnier D, Yadan JC, Chaudière J (1998) Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol 11: 1184-1194.
-
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329: 23-38.
-
(2014) General Law of Health and the Area of Clinical Research for Health. Mexico: Official Gazette.
-
World Medical Association Inc (2009) Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc 107: 403-405.
-
(1997) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Int Dig Health Legis 48: 231-234.
-
Kumar A, Sharma S, Pundir CS, Sharma A (1995) Decreased plasma glutathione in cancer of the uterine cervix. Cancer Lett 94: 107-111.
-
Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6: 45-57.
-
Demirci S, Ozsaran Z, Celik HA, Aras AB, Aydin HH (2011) The interaction between antioxidant status and cervical cancer: a case control study. Tumori 97: 290-295.
-
Manju V, Kalaivani Sailaja J, Nalini N (2002) Circulating lipid peroxidation and antioxidant status in cervical cancer patients: a case-control study. Clin Biochem 35: 621-625.
-
Beevi SS, Rasheed MH, Geetha A (2007) Evidence of oxidative and nitrosative stress in patients with cervical squamous cell carcinoma. Clin Chim Acta 375: 119-123.
-
Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S64-73.
-
Jia Y, Hu T, Hang CY, Yang R, Li X, et al. (2012) Case-control study of diet in patients with cervical cancer or precancerosis in Wufeng, a high incidence region in China. Asian Pac J Cancer Prev 13: 5299-5302.
-
González CA, Travier N, Luján-Barroso L, Castellsagué X, Bosch FX, et al. (2011) Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. Int J Cancer 129: 449-459.
-
Myung SK, Ju W, Kim SC, Kim H; Korean Meta-analysis (KORMA) Study Group (2011) Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: a meta-analysis. BJOG 118: 1285-1291.
-
Cox BA, Crow WT, Johnson L (2012) Current nutritional considerations for prevention of cervical cancer. Osteopath Fam Physician 4: 81-84.
-
Cunzhi H, Jiexian J, Xianwen Z, Jingang G, Shumin Z, et al. (2003) Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res 94: 113-122.
-
Greenlee H, White E, Patterson RE, Kristal AR; Vitamins and Lifestyle (VITAL) Study Cohort (2004) Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. J Altern Complement Med 10: 660-666.
-
Flood A, Peters U, Chatterjee N, Lacey JV Jr, Schairer C, et al. (2005) Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev 14: 126-132.
-
Hosono S, Matsuo K, Kajiyama H, Hirose K, Suzuki T, et al. (2010) Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur J Clin Nutr 64: 400-409.
-
Wang JL, Lin YW, Chen HM, Kong X, Xiong H, et al. (2011) Calcium prevents tumorigenesis in a mouse model of colorectal cancer. PLoS One 6: e22566.
-
Lamprecht SA, Lipkin M (2003) Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 3: 601-614.
-
Dougherty U, Mustafi R, Wang Y, Musch MW, Wang CZ, et al. (2011) American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: role of EGFR. BMC Complement Altern Med 11: 111.





