Journal of Clinical Nephrology and Renal Care
Patient Perception of Treatment Success: A Qualitative Interview Study in Patients with End-stage Renal Disease undergoing Hemodialysis
Anna Rydén1*, Matthew Wolfe2 and Mona L Martin3
1AstraZeneca R & D, Pepparedsleden 1, Mölndal, Sweden
2Formerly of Health Research Associates, Seattle, USA
3Health Research Associates, Seattle, WA 98043, USA
*Corresponding author:
Anna Rydén, AstraZeneca R & D, Pepparedsleden 1, SE-431 83 Mölndal, Sweden, E-mail: anna.ryden@astrazeneca.com
J Clin Nephrol Ren Care, JCNRC-2-005, (Volume 2, Issue 1), Research Article
Received: December 08, 2015: Accepted: January 28, 2016: Published: February 01, 2016
Citation: Rydén A, Wolfe M, Martin ML (2016) Patient Perception of Treatment Success: A Qualitative Interview Study in Patients with End-stage Renal Disease undergoing Hemodialysis. J Clin Nephrol Ren Care 2:005
Copyright: © 2016 Rydén A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:End-stage renal disease treated with hemodialysis (ESRD-HD) is associated with poor outcomes for patients and can substantially reduce health-related quality of life (HRQL). Alongside drug development for this patient group, there is a need for a better understanding of patients' perceptions of their condition, their symptoms and the impact ESRD-HD has on their lives. Here, we report data from qualitative, semi-structured, concept-elicitation interviews, conducted as part of a phase 2 clinical study of the sodium-hydrogen exchanger isoform 3 (NHE3) inhibitor tenapanor for the treatment of fluid overload in patients with ESRD-HD.
Methods:Patients enrolled in the clinical trial (NCT01764854) were invited to participate in the interviews, which were structured to focus on symptoms before moving on to the impact of the disease on HRQL. Patients' expressions of ESRD-HD-related concepts were identified in the interview transcripts and coded to allow each concept to be classified into sub-domains. Patients' perceptions of treatment success were also assessed using open-ended questions.
Results:Overall, 21 patients (12 men) participated in these interviews (mean age ± standard deviation, 51.4 ± 12.8 years). Energy-related, digestive and dryness-related symptoms, and pain and discomfort were the predominant symptom concepts identified from the patients' expressions. The necessity of adopting coping behaviors (with concepts such as reducing water consumption and dietary restrictions), emotional difficulties (such as irritability, aggravation and agitation), and social/lifestyle limitations and restrictions (such as restricted travel activities) were identified as key sub-domains impacted by the disease. When discussing treatment success, patients focused on not needing to have so much fluid removed during hemodialysis, having fewer adverse effects from hemodialysis and experiencing less fluid retention.
Conclusions:These qualitative interviews in patients with ESRD-HD and fluid overload provide valuable information on patients' perceptions of their disease. The study findings may be useful in supporting content validity when developing future or modifying existing patient-reported outcome instruments suitable for this patient group.
Keywords
Chronic kidney disease, End-stage renal disease, Hemodialysis, Impact of disease, Patient-reported outcomes, Symptoms, Treatment
Abbreviations
ESRD: end-stage renal disease; ESRD-HD: end-stage renal disease treated with hemodialysis; HRQL: health-related quality of life; PRO: patient-reported outcome.
Background
End-stage renal disease (ESRD, also known as chronic kidney disease stage 5) is a major public health burden worldwide. According to the annual report of the European Renal Association-European Dialysis and Transplant Association Registry, the prevalence of ESRD at the end of 2012 was in the range 683-1251 cases per million population across 17 European countries [1]. The 2013 report of the US Renal Data System (USRDS) indicated that the prevalence of ESRD in the USA was 1924 cases per million population at the end of 2011 [2]. Hemodialysis is the most common treatment for patients with ESRD, followed by peritoneal dialysis and pre-emptive kidney transplantation. Patients with ESRD who are treated with hemodialysis (ESRD-HD) experience poor health-related quality of life (HRQL) and outcomes [3-5]. There is therefore an ongoing need for new therapies to improve long-term outcomes and HRQL for patients with ESRD-HD.
When investigating health outcomes during the development of new medicines, it is important to gain an understanding of the patients' perspective. Patient-reported outcomes (PROs) are often used as endpoints in clinical trials; therefore, their usefulness and relevance to patients (i.e. the content validity) should be demonstrated [6]. The symptom burden of chronic kidney disease has been the subject of a recent review of the literature [7]. One conclusion of this review was that the instruments used for collecting PROs were varied and often failed to capture fully those symptoms important to patients. This highlights a need for an improved understanding of the symptoms experienced by patients with chronic kidney disease, which could assist in developing new or modifying existing PRO instruments and in identifying avenues for improving patient care. An important step in developing PRO instruments is ensuring content validity; using qualitative interviews to gather perceptions directly from patients is an important part of this process [8].
Tenapanor (AZD1722) is a first-in-class, minimally systemic, small-molecule inhibitor of the sodium-hydrogen exchanger isoform 3 (NHE3) [9]. Tenapanor is under development for the treatment of patients with chronic kidney disease. It has been evaluated for treating fluid overload in individuals with ESRD-HD in a phase 2 studies, which included patient interviews designed to investigate and identify condition-specific concepts that are relevant to patients with ESRD-HD and fluid overload. Here, we report data from this exploratory, qualitative study. The primary aim of the interviews was to evaluate the experiences of patients regarding symptoms and the impact their condition has on their daily HRQL, and to identify participants' perceptions about the definition of successful treatment.
Methods
Participants
Patients with ESRD-HD and fluid overload who had been formally enrolled in this US-based clinical trial of tenapanor (ClinicalTrials.gov identifier, NCT01764854) were invited to participate in the qualitative interviews. Each interested patient was screened briefly by telephone to confirm their interest in participating and to schedule a time during the run-in phase to conduct the pre-treatment telephone interview. Interviews were conducted between March and November 2013. Written informed consent for participation in the study was obtained from participants.
Data collection
This study was performed in accordance with the ethical principles of the Declaration of Helsinki and appropriate Institutional Review Board approval was obtained before the study commenced. All data collected in this study were strictly confidential in accordance with local, state and federal law.
Interview design and structure
The concept-elicitation interviews were structured to focus initially on symptoms of ESRD before moving on to impact of the disease. The process began with open-ended questions such as "what symptoms do you have that are related to your kidney disease?" followed by specific probes designed to explore further the various aspects of each symptom identified by patients. The interview proceeded to topics about expectations of additional treatment for their condition, using open-ended questions such as "can you describe your current thoughts about what makes treatment successful?". These were followed by questions investigating the changes in symptoms and impacts on daily life that patients would like to experience as a result of treatment.
The interviews lasted approximately 30-45 minutes and were conducted by three trained interviewers. All interviewers were skilled in conducting individual patient interviews for PRO measurement development. Interviews were recorded and the digital audio files of interviews were sent to a professional transcription company.
Data analysis
Data collection and analysis for this study were carried out according to the 'best practice' recommendations for establishing the content validity of PRO instruments used in the evaluation of medical products [8,10,11]. A coding framework was developed at the outset of the process. Patient expressions of ESRD-HD-related symptoms and impact of the condition on their HRQL were identified in the interview transcripts; two coders were used to assign codes to expressions. ATLAS.ti software (version 7.0; Cleverbridge, Chicago, IL, USA) was used to group codes arising from similar content into sub-domains. The coding framework was revised as necessary during the process to reflect the content identified in the transcript database.
To assess 'saturation of concept', transcripts were ordered chronologically and then divided into quintiles. Codes assigned to each transcript quintile were compared with the codes recorded for the previous quintile. Saturation was reached when no new codes were identified, indicating that all concepts forthcoming from the study population were captured within the existing transcript database and data from additional interviews were not likely to contribute further information.
In order to assess the consistency with which codes were assigned, an analysis of inter-rater agreement was conducted. Four randomly selected transcripts were independently coded by both coders and the coding was then compared. Consistency of coding was characterized by the percentage of agreement in both the identification of concepts and the assignment of codes to each identified concept.
Results
Patients
Ninety patients with ESRD-HD who were identified at 12 sites consented to be contacted regarding potential participation in the interviews. Some of these patients could not subsequently be interviewed for reasons such as a lack of fluency in the English language, not meeting the clinical trial inclusion criteria after initial screening or being unable to be contacted during the run-in phase. In total, 21 patients from eight sites completed the interview part of the study and were included in the analysis.
The mean (± standard deviation) age of the interviewees was 51.4 (± 12.8) years (range, 28-79 years) and there were more men (n = 12) than women. Patients had been undergoing hemodialysis for a mean (± standard deviation) of 7.1 (± 4.2) years (range, 1-18 years). Causes of ESRD in these patients included hypertensive nephrosclerosis (n = 5), diabetic nephropathy (n = 3) and polycystic kidney disease (n = 2).
Saturation and agreement
Saturation of concepts was achieved after the third quintile, except for one concept in the fourth quintile ('fever'), which was described by a single patient and was not thought to be related to ESRD. Inter-rater agreement was high: 82-91% for the identification of expressed concepts and 97-100% for the assignment of codes.
Expressions relating to symptoms
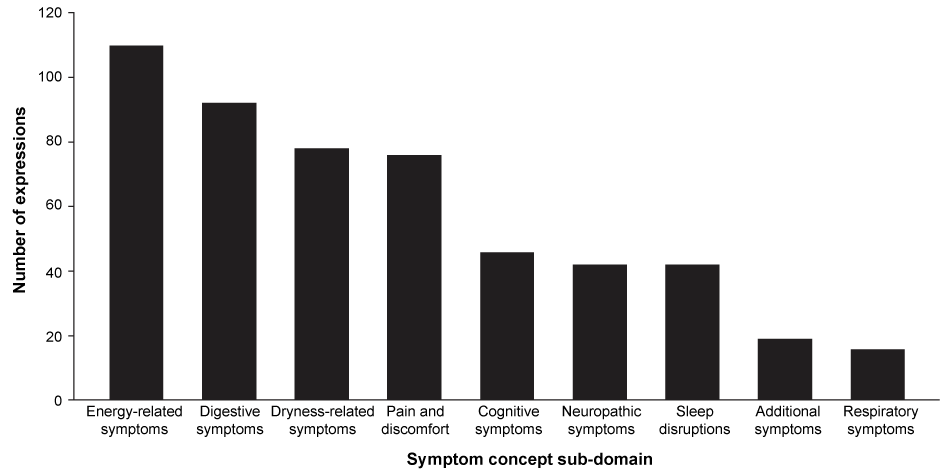
A total of 521 expressions describing symptoms were identified in the transcripts. Symptom concepts most commonly fell within the sub-domains of 'energy-related', 'digestive' and 'dryness-related' symptoms and 'pain and discomfort' (Figure 1). Table 1 shows the frequency of symptom concepts (i.e. the number of patient expressions coded for each concept) within the transcripts. The number of patients (or transcripts) contributing to each concept is also shown. The predominant symptom concepts expressed were general tiredness, thirst, muscle cramping and physical fatigue or tiredness, which were reported 68 (13%), 41 (8%), 38 (7%) and 30 (6%) times, respectively; 62-71% of patients contributed to these concepts.
.
Figure 1: Number of symptom concept expressions in the transcripts for each symptom sub-domain (total number of expressions = 521; 21 patients included in study).
View Figure 1
![]()
Table 1: Summary of symptom concept expression frequencies.
View Table 1
Expressions relating to impact of disease
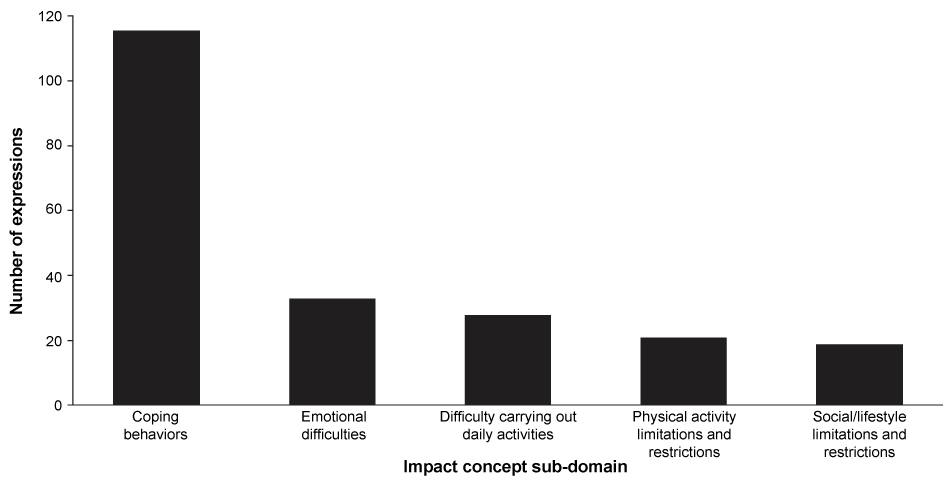
A total of 217 expressions describing the aspects of life most affected by ESRD-HD were recorded in the transcripts. The sub-domain 'coping behaviors' captured most of the impact expressions (116 expressions; Figure 2). The most frequently expressed types of coping behavior were 'reducing water consumption' (28 expressions, 13% of all impact expressions), 'dietary restrictions' (25 expressions, 12%) and 'maintaining a positive outlook' (18 expressions, 8%) (Table 2). Other areas that patients often reported as being affected by ESRD-HD fell into the sub-domains of 'emotional difficulties' or 'social/lifestyle limitations and restrictions', with 'irritability, aggravation and agitation' (17 expressions, 8%) and 'restricted travel activities' (12 expressions, 6%), respectively, being the most commonly mentioned concepts.
.
Figure 2: Number of impact concept expressions in the transcripts for each impact sub-domain (total number of expressions = 217; 21 patients included in study).
View Figure 2
![]()
Table 2: Summary of impact concept expression frequencies.
View Table 2
Patient definitions of treatment success
During their interview, patients were asked how they would define successful treatment for ESRD-HD. The most common themes are shown in table 3 with examples of patient quotes. Reductions in the amount of fluid being removed during hemodialysis, experiencing fewer side effects from hemodialysis and less fluid retention were key themes. When asked how their symptoms would have to change in response to treatment for them to describe it as successful, improvements in symptoms related to fluid retention, constipation, energy and thirst were themes expressed by patients. Most individuals were unable to provide a response when asked what change in the impact of their condition would need to occur in order for a treatment to be described as successful; of those who did respond, increased activity level was the emergent theme.
![]()
Table 3: Patient responses to interview questions about successful treatment.
View Table 3
Discussion
In this qualitative exploratory study of individuals with ESRD-HD with fluid overload, a thorough interview technique was used to elicit patient concepts of condition-specific symptoms and impact of disease, as well as identifying patient perceptions of successful treatment. The results showed that general tiredness, thirst, muscle cramping and physical fatigue were the symptoms most often expressed by patients. Patients identified the necessity to adopt coping behaviors (e.g. restrictions to diet and water consumption, and to maintain a positive outlook) as the main impact their condition had on HRQL. Other predominant impacts were irritability, aggravation and agitation, and restricted travel activities. Patients considered that a successful treatment would be one that reduced the amount of fluid being removed during hemodialysis, reduced the number of side effects from hemodialysis and reduced fluid retention between dialysis sessions.
The symptoms reported by patients in our study are broadly consistent with those previously described by patients with ESRD as being common, such as fatigue/lack of energy and pain [12]. The results help to illustrate the impact of ESRD, fluid overload and hemodialysis treatment on HRQL. The need to assess and implement measures for improving HRQL in patients with ESRD has been recognized [4]; depression has been reported to occur in 25-30% of patients [4], with symptoms of depression being independently associated with increased mortality in patients who have received long-term dialysis [3-5].
The results of this study could potentially provide a useful basis for initial development of a new PRO instrument, or modification of an existing instrument, for use in patients with ESRD-HD and fluid overload. PROs have an important role to play in evaluating medicines. Traditionally, physical, physiological and biochemical measures of disease activity have been employed to evaluate the pharmacological effects of drug therapy. However, they do not reflect patients' perceptions of function and well-being. Yet, these perceptions are largely responsible for whether a patient considers they are benefiting from a treatment [13]. Studies show only modest correlations of physiological measures and symptoms with functional capacity and well-being [13].
Previously, PRO instruments have been developed via multidisciplinary collaboration among healthcare providers, followed by psychometric evaluation of draft instruments. Little or no input from the patient has been sought and instruments have often been complicated, poorly understood or not entirely relevant to the target patient population. Obtaining qualitative data, collected via interviews such as those used in our study, is now considered an important step in the process of establishing the content validity of a PRO instrument, according to 'best practice' recommendations for developing PRO instruments used in the evaluation of medical products [8,10,11]. We have designed our study based on these principles, and have addressed concepts which are relevant to patients, as well as saturation of concept and inter-rater agreement. Content validity requires multiple forms of evidence, including that the concepts selected for a measure are supported by the literature, are important to clinicians in assessing the condition, and are relevant and important to patients [8]. In this paper, we have provided support for concepts that are relevant and important to patients.
The need for PRO instruments that best serve the needs of patients with advanced chronic kidney disease (stages 4 or 5) has been documented in a systematic review by Almutary et al. in 2013 [7]. The review showed considerable variation in the PRO instruments used across the studies identified. The two most commonly used PRO instruments among patients with advanced chronic kidney disease were the Dialysis Symptom Index (DSI) [14] and the Memorial Symptom Assessment Scale (MSAS) short form (MSAS-SF) [15]. The MSAS was originally developed by Portenoy et al. [16] for use in patients with advanced cancer. The MSAS consists of 32 symptoms; patients record the presence of each symptom, as well as the frequency and distress caused by each symptom (i.e. the 'symptom dimensions'). This facilitates the detailed capture of the overall symptom experiences of the patients. Chang et al. [15] subsequently developed the MSAS-SF to reduce patient burden by removing most of the symptom dimension parameters. The MSAS-SF has been further modified for use in patients with chronic kidney disease by the addition of several renal symptoms [17-19]. The DSI [14], which measures 30 symptoms, was derived from the MSAS-SF. Hence, not only is the DSI based on an instrument designed for patients with a different disease, it also does not capture symptom dimension adequately. This was illustrated by a modification of the DSI by Danquah et al. [20] who added two symptom dimension parameters (frequency and intensity). The importance of fully capturing the frequency, intensity and distress of symptoms to characterize the overall symptom experience of patients adequately has been highlighted by Armstrong [21].
Patients with ESRD are likely to have several comorbidities and may therefore experience additional symptoms that are not present in those with less advanced chronic kidney disease. These differences in symptom burden present challenges for a 'one size fits all' approach regarding PRO instruments for patients with chronic kidney disease and support the case for selection of the most relevant symptoms, impacts and treatment expectations for the target population when developing a PRO instrument. The latter approach, which we have used in our study, should facilitate the crafting of a PRO instrument that is content valid with respect to the intended target patient population since more research needs to be done on methods to alleviate the identified symptoms in this patient population.
The 21 patients enrolled in our study had broadly similar disease characteristics to the general ESRD population described in the 2013 report of the USRDS [2], although they were slightly younger (mean age in our study was 51.4 years versus 61.2 years for the USRDS population [2]). It is important to note, however, that the results of our study are not intended to be representative of the general population of patients with ESRD-HD, but instead are intended to mirror those of patients with ESRD-HD with fluid overload participating in future clinical trials. Since our study targeted individuals with ESRD-HD and fluid overload, it may be expected that they would focus on fluid-related symptoms with regard to concepts of treatment success. Any bias that might occur from self-selection or patient willingness to participate can be expected to be no different than that found in any clinical trial or observational study.
Conclusions
This study describes the experiences, condition-specific symptoms and impact of disease on HRQL in patients with ESRD-HD and fluid overload. It also identifies patient-perceived concepts of treatment success. Energy-related, digestive and dryness-related symptoms, and pain and discomfort were the predominant symptom concepts expressed by patients. The need to adopt coping behaviors was the biggest impact of disease on HRQL. Patients felt that key elements of successful treatment should be reducing the amount of fluid removed during hemodialysis, reducing the number of side effects from hemodialysis and reduced fluid retention between dialysis sessions. Our study was conducted in accordance with the best practice guidelines for establishing content validity in PRO instruments [8,10,11]. Our findings provide guidance for the development of future PRO instruments suitable for patients with ESRD-HD and fluid overload and help to identify avenues for improving their care and HRQL.
Competing Interests
AR is an employee of AstraZeneca R & D, Mölndal, Sweden. MW is a former employee of Health Research Associates Inc., Seattle, Washington, USA. MLM is an employee of Health Research Associates Inc., Seattle, Washington, USA.
Authors' Contributions
All three authors were involved in the design of the study and analysis and interpretation of the data. All three authors provided direction regarding the content of the manuscript at all stages of its development and approved the final version.
Acknowledgments
We thank all the patients, investigators and interviewers who participated in this study, and the analysts who were involved in evaluation of the study data. We thank Dr Steven Inglis (PhD) of Oxford PharmaGenesis who provided medical writing support that was funded by AstraZeneca R & D, Mölndal, Sweden.
Funding
This study was funded by AstraZeneca.
References
-
European Renal Association (2012) European Dialysis and Transplant Association (ERA-EDTA) Registry: Annual report.
-
United States Renal Data System (2013) Annual Data Report, Atlas of CKD & ESRD.
-
Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV5 (2014) Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis 63: 623-635.
-
Finkelstein FO, Wuerth D, Finkelstein SH (2009) Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int 76: 946-952.
-
Hedayati SS, Bosworth HB, Briley LP, Sloane RJ, Pieper CF, et al. (2008) Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 74: 930-936.
-
Food and Drug Administration (2009) Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims.
-
Almutary H, Bonner A, Douglas C (2013) Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 39: 140-150.
-
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, et al. (2011) Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value Health 14:967–977.
-
Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, et al. (2014) Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36.
-
Brod M, Tesler LE, Christensen TL (2009) Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res 18: 1263-1278.
-
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, et al. (2011) Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2--assessing respondent understanding. Value Health 14:978–988.
-
Murtagh FE, Addington-Hall J, Higginson IJ (2007) The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis 14: 82-99.
-
Chassany O, Sagnier P, Marquis P, Fullerton S, Aaronson N, et al. (2002) Patient-Reported Outcomes: The Example of Health-Related Quality of Life—A European Guidance Document for the Improved Integration of Health-Related Quality of Life Assessment in the Drug Regulatory Process. Therapeutic Innovation & Regulatory Science 36: 209-238.
-
Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, et al. (2004) Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage 27: 226-240.
-
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000) The memorial symptom assessment scale short form (MSAS-SF). Cancer 89: 1162-1171.
-
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, et al. (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30A: 1326-1336.
-
Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, et al. (2006) Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med 20: 631-636.
-
Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, et al. (2007) Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 10: 1266-1276.
-
Murtagh FE, Addington-Hall J, Edmonds P, Donohoe P, Carey I, et al. (2010) Symptoms in the month before death for stage 5 chronic kidney disease patients managed without dialysis. J Pain Symptom Manage 40: 342-352.
-
Danquah FV, Zimmerman L, Diamond PM, Meininger J, Bergstrom N (2010) Frequency, severity, and distress of dialysis-related symptoms reported by patients on hemodialysis. Nephrol Nurs J 37: 627-638.
-
Armstrong TS (2003) Symptoms experience: a concept analysis. Oncol Nurs Forum 30: 601-606.