International Journal of Transplantation Research and Medicine
A Complicated Case of Transplant Renal Artery Stenosis: A Case Report and Literature Review
Gregor Mlinsek1, Alexander Jerman1, Damjan Kovac1, Jelka Lindic1, Miro Mihelic2, Nikola Lakic3, Aljosa Kandus1 and Jernej Pajek1*
1Department of Nephrology, University Medical Centre Ljubljana, ZaloSka 7, 1000 Ljubljana, Slovenia
2Department of Urology, University Medical Centre Ljubljana, ZaloSka 7, 1000 Ljubljana, Slovenia
3Department of Cardiovascular Surgery, University Medical Centre Ljubljana, ZaloSka 7, 1000 Ljubljana, Slovenia
*Corresponding author:
Jernej Pajek, Department of Nephrology, University Medical Centre Ljubljana, Zaloska 7, 1000 Ljubljana, Slovenia, Email: jernej.pajek@mf.uni-lj.si
Int J Transplant Res Med,
IJTRM-1-010, (Volume 1, Issue 2),
Case Report
Received: September 18, 2015: Accepted: October 19, 2015: Published: October 22, 2015
Citation: Mlinsek G, Jerman A, Kovae D, Lindie J, Mihelie M, et al. (2015) A Complicated Case of Transplant Renal Artery Stenosis: A Case Report and Literature Review. Int J Transplant Res Med 1:010
Copyright: © 2015 Mlinsek G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Transplant renal artery stenosis (TRAS) is a well recognized complication of kidney transplantation. Hypertension with or without an increasing creatinine level is the most common presentation. Recognition of TRAS is important because it represents a potentially reversible cause of hypertension, allograft loss and adverse patient outcome. Here we report a demanding case of TRAS in a kidney transplant recipient who received a pediatric kidney. The complicated course included conservative therapy, PTRA and surgical revascularization, bringing the attention to several infrequently reported issues in the management of this transplant complication.
Keywords
TRAS: Transplant Renal Artery Stenosis, Goldblatt kidney, Conservative therapy, PTRA, Surgical revascularization
Introduction
Transplanted renal artery stenosis (TRAS) is the most frequent vascular complication in renal transplantation with an incidence varying between 1 - 23% [1-4]. Some authors reported a higher incidence of TRAS in transplants from cadavers (13.2 - 17.7%) compared to live donors (1.3 - 5.8%) [5], whereas others found a higher incidence after living kidney transplantation [6]. TRAS significantly affects the long term graft outcome [7]. The reported frequency of TRAS depends on the criteria used to define stenosis, i.e. peak systolic velocity (PSV) measured by Duplex-Doppler [8] or degree of stenosis seen on computer tomography angiography (CTA), magnetic resonance angiography (MRA) or intravenous angiography. PSV usually reported to be associated with significant stenosis is 1.5 - 3.0 m/s [3,9-11], frequently 2.0 m/s or more [12,13]. Structural equivalent to these PSV values is 50 to 70% reduction of the arterial diameter [4] or as can be calculated and expressed in surface, 75 to 91% reduction of arterial cross section, respectively. More important than PSV or arterial lumen reduction, however, are (1) intrarenal parameters ("parvus et tardus" pulse wave shape, resistance index - RI, acceleration time - AT and acceleration index - AI) that reflect hemodynamic effect and thus importance of the stenosis and (2) arterial hypertension, edema formation, renal transplant failure and potential graft loss representing clinical manifestation of hemodynamically important renal stenosis.
TRAS can develop due to various causes, at different sites of the renal artery and at different time after transplantation. It may develop proximal to the anastomosis (pre-anastomotic), at the anastomosis or within the donor artery (post-anastomotic). In pre-anastomotic (proximal) TRAS intrarenal hemodynamic changes result from iliac artery stenosis. TRAS is most frequently a consequence of surgical procedure or preservation techniques, i.e. damage during nephrectomy, due to vascular clamps injury, cannulation for organ perfusion, traction of the vessels, suture techniques, torsion, kinking or angulation if the artery is longer than the vein or otherwise redundant. End to side anastomoses are associated with lower incidence of TRAS than end to end anastomoses [14]. Ostial stenosis is less frequent with a Carrel patch [15]. Other reasons are atherosclerosis in donor renal or recipient iliac artery. Some authors report acute rejection, CMV infection and delayed graft function to be associated with TRAS [16,17], while others found no association of TRAS with prolonged cold ischemia time, acute rejection or cytomegalovirus status [2]. Parameters like CaPO4 product, serum LDL cholesterol and perhaps uric acid may increase the risk of TRAS development late after renal transplantation [18]. TRAS may appear any time after transplantation, most frequently however between 2 - 45 months after transplantation [1,2]. Stenosis due to mechanical reason appears earlier in the post-transplant course. There are three therapeutic options to treat TRAS, i.e. medical treatment, percutaneous transluminal renal angioplasty with or without stenting and surgical revascularization. A recent study comparing different endovascular intervention (EVI) types (DES: drug-eluting stent, BMS: bare-metal stent or only PTA: Percutaneous Transluminal Angioplasty) found a significant improvement in allograft function and mean arterial blood pressure with no significant differences among EVI types. There was also no significant difference in allograft survival with respect to EVI types [19]. There was no difference in allograft survival between renal allograft without TRAS and those with TRAS treated by angioplasty [20].
Case Report
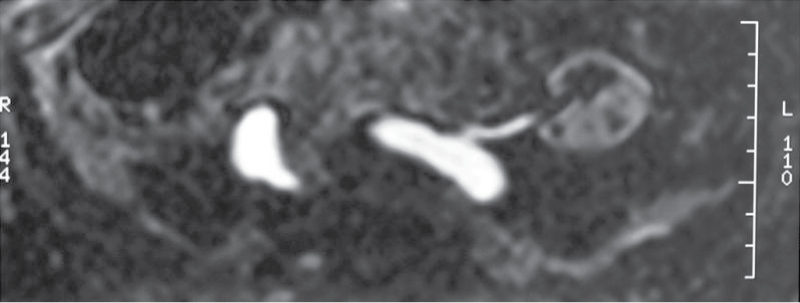
A 66-year-old male kidney transplant recipient was admitted to our hospital in august 2010 due to the worsening of allograft function, edema of lower extremities and signs of decompensated heart failure. He has been treated for hypertension for over 20 years. Since 1990 when ESRD due to hypertensive nephroangiosclerosis was diagnosed, he was treated by chronic hemodialysis. In 2005 he got transplanted a deceased child donor kidney. The child who was 5.5 years old died due to hypoxic brain injury caused by drowning. Cold ischemia time was 19 hours, kidney measured 87 mm in length, donor pre-terminal creatinine value was 53 μM and there was a single donor artery in patch. Anastomosis type was end to site. Although surgery was uneventful, diuresis in the early postoperative course did not appear. Multiple Doppler ultrasound (US) examinations revealed good transplant perfusion with a turbulent flow and a peak systolic value/velocity (PSV) > 2 m/s at the anastomosis. Surgical revision was performed and stenosis at the anastomosis was excluded. Narrow renal transplant artery, subsequently shown also by magnetic resonance angiography - time of flight sequences (MRA-TOF), was confirmed (Figure 1). Arterial diameter was 3 mm only. Although the narrow artery was patent, the kidney perfused and continuous furosemid infusion (1g/day) administered several hours after the transplantation the patient remained anuric for 49 hours. Thereafter brisk diuresis appeared, reaching up to 500 ml/hour. In the meantime he needed one hemodialysis procedure. He was discharged with a creatinine 203 μM which dropped to < 100 μM several months after the transplantation. Induction immunosuppression was with basiliximab later maintained by cyclosporin A, methylprednisolone and mycophenolate mofetil.
.
Figure 1: Transplant renal artery stenosis - due to a narrow child artery shown by MRA TOF sequences.
View Figure 1
In February 2007, one year and a half after the transplantation, he presented with raised serum creatinine levels (116 μM). Doppler US and MRA both showed a hemodynamically significant narrowing at the ostium of transplanted renal artery. A percutaneous transluminal renal angioplasty (PTRA) was performed. The intervention, however, did not succeed to dilate the stenosis significantly. Postoperatively some of the Doppler indices (AI, PSV) were even worse than before the intervention, although post-procedural serum creatinine dropped to 78 μmol/L. Blood pressure, controlled with antihypertensives, was normal. We therefore decided to continue with conservative therapy. Clopidogrel 75 mg daily was added to his regular therapy to prevent thrombosis at TRAS (Table 1).
![]()
Table 1: Doppler ultrasound indices pre-PTRA and post-PTRA.
View Table 1
The patient was discharged and regularly seen in the outpatient setting with relatively stable values of serum creatinine (range 78 to 135 μmol/L) until 2010, when he was hospitalized due to the signs of heart failure. Heart failure was believed to be associated with the aortic stenosis, which was diagnosed previously. In the first instance he was treated with parenteral diuretic and lost 7 kg of body weight in a few days with much improvement in his symptoms. He regained 5 kg of body weight in the next few months and presented for the second time with resistant hypertension, worsened peripheral edema, dyspnea and elevated serum creatinine. At that time he was inefficiently treated with 7 antihypertensive drugs (diltiazem, carvedilol, nifedipine, moxinidine, furosemide, doxazosin and spironolactone).
An ECG revealed atrial fibrillation with the heart rate of 80 beats per minute, chest radiography reported increased heart size with probability of left atrium enlargement and signs of pulmonary hypertension. There was a marked lung interstitial edema with small amount of fluid in right pleural space. Echocardiography revealed concentric hypertrophy of the left ventricle with good left ventricle systolic function. Left atrium was moderately dilated. There was aortic stenosis with maximal transvalvular gradient 55 mm Hg and estimated aortic valve area of 1.1 cm2. There were no signs of aortic regurgitation. Mitral and tricuspid regurgitation were moderate. Estimated systolic pressure in the right ventricle was 40 mm Hg + CVP.
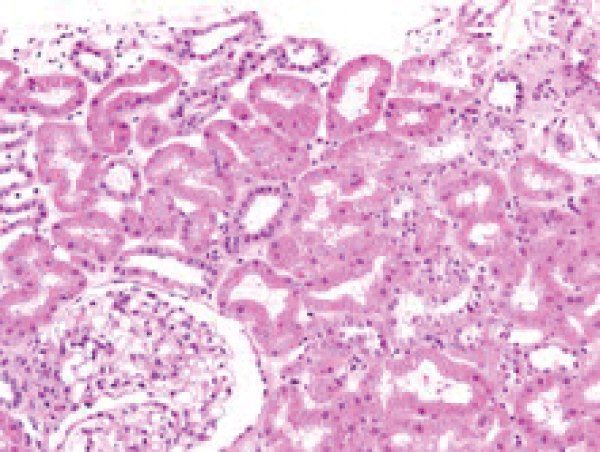
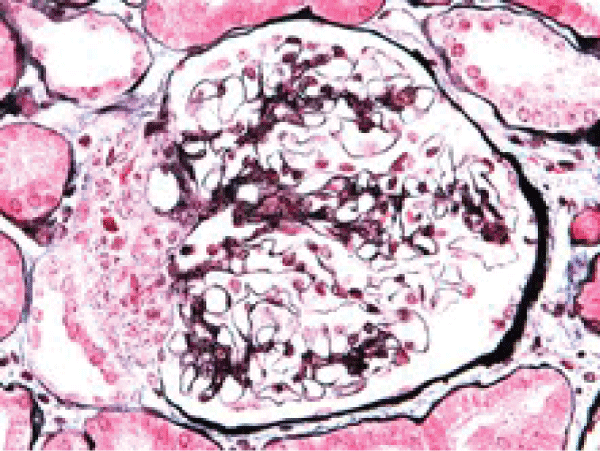
Renal ultrasound showed a norma transplanted kidney size (12 cm), normal parenchyma (13 mm) and no dilatation of collecting system. As intrarenal Doppler US indices with "parvus et tardus" flow contour were not substantially different from those obtained in the year 2007, see Table 1), transplant rejection as a cause for worse graft function seemed possible. Kidney biopsy was performed. Pato-hystological examination revealed Goldblatt-nephropathy-like changes. There was juxtaglomerular focal hyperplasia with electron microscopy confirmed renin-like granules (Figure 2a and Figure 2b). A multifocal mononuclear-cell infiltration was present, however the criteria for cellular rejection were not met and also negative C4d in peritubular capillaries and negative HLA-DR in proximal tubules supported the exclusion of transplant rejection as a cause of renal function deterioration.
.
Figure 2a: Hyperplastic juxtaglomerular apparatus at the glomerular hilum (HE staining).
View Figure 2a
.
Figure 2b: Hyperplsia of juxtaglomerular apparatus cells between glomerular hilum and macula densa. In multiplied cells there are argirophyl granula, which on EM show granula of renine type (PASM and Azan staining).
View Figure 2b
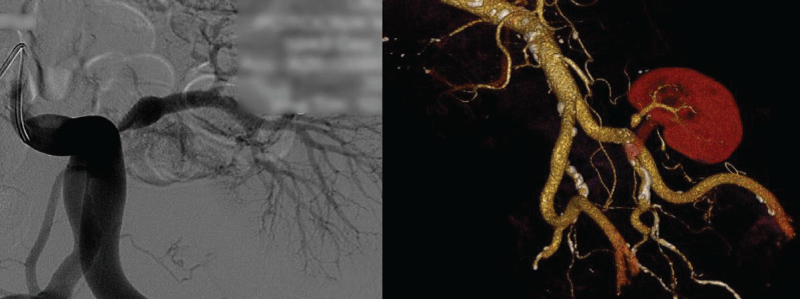
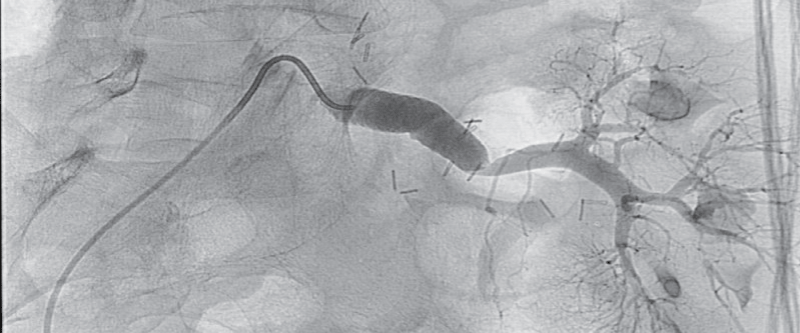
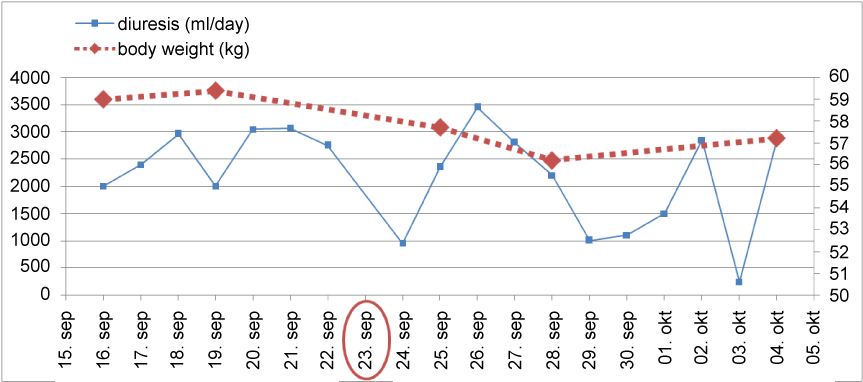
Thereafter pelvic arteriography showed hemodynamically significant transplant artery stenosis at anastomosis with post-stenotic dilatation (Figure 3). Given the patient's history of previous dilatation attempt in 2007, a decision for surgical bypass of renal artery stenosis was made, using a 8 mm Dacron graft to anastomose left iliac common artery and distal post-stenotic part of transplant renal artery (Figure 4). The day after surgical procedure brisk diuresis appeared (Graph 1). Polyuria was accompanied by body weight reduction. Elevated serum creatinine fell to normal range (113 to 82 μmol/l). On day 5 the patient developed severe orthostatic hypotension (75/50 mm Hg) which resolved after discontinuation of all antihypertensive drugs and administration of higher hydrocortisone dose. Blood pressure stabilized at normal values after 6 days. On discharge he was slightly hypotensive with blood pressure 101/84 mm Hg, without any antihypertensive drugs and creatinine value 67 μM, body weight 57.7 kg and without leg edema.
.
Figure 4: 8 mm Dacron stent graft anastomosis between left iliac common artery and transplant renal artery.
View Figure 4
On follow-up the patient was improving until April 2011, when he again presented with worsened symptoms: hypertension (RR 172/120 mm Hg), recurrent leg edema, elevated serum creatinine level (118 μmol/l) and body weight (70.0 kg). Ultrasound revealed relative stenosis on iliorenal bypass anastomosis. CT angiography reported hemodynamically important stenosis on renal transplant artery (as expected, for this was one reason for ileorenal bypass procedure in September 2010) and a stenosis on distal segment on ileorenal bypass was proposed. PTA with balloon stenting (8 × 27 mm) within the distal segment of the bypass was performed in August 2011. Two days after procedure he was discharged with serum creatinine level 165 μmol/l and body weight 72.8 kg. However, in subsequent course creatinine levels and body weight rose. No further attempt to resolve the stenosis was made.
.
Graph 1: Diuresis and body weight in the perioperative period. Encircled in red is the operation day.
View Graph 1
Discussion
PSV and detection of hemodynamically important stenosis in pediatric kidney transplants
The question is whether or not PSV of more than 2 m/s, which indicates, as generally believed, severe and hemodynamically important TRAS in adult population, calls for intervention in a transplanted pediatric kidney?. It seems that PSV in this setting can be of only relative significance and without a high predictive value. If interpreted without concurrent clinical and laboratory data it can even be deceiving. Such kidneys have higher velocities not only at the time of transplantation but also several years afterwards. In a recent study mean maximal PSV for the pediatric transplants was 2.0 (0.9 - 3.8) m/s, which was almost twice the mean value of 1.1 (0.7 - 1.6) m/s for the adult kidneys [21]. A PSV > 1.8 m/s itself did not necessarily indicate TRAS in pediatric kidneys transplanted to adult recipients. Only a minority of patients with elevated systolic velocities had hemodynamically significant stenosis [21]. Furthermore, not only pediatric kidneys transplanted to adults have increased PSV but also those transplanted to children [10]. Pediatric transplant artery was reported to show PSV of 0.9 - 6.1 m/s but they only rarely had clinically significant stenosis which was demonstrated by increased creatinine. There was no correlation between PSV and serum creatinine nor with blood pressure in pediatric population. Other parameters, such as consistently elevated blood pressure, creatinine or proteinuria may indicate TRAS in pediatric population rather than relying on Doppler characteristics alone [10]. In our case early high PSV together with anuria suggested hemodynamically important stenosis which was the reason for surgical exploration soon after transplantation, which however showed only a narrow renal artery. We conclude that increased PSV in pediatric artery may not reflect a hemodynamically important TRAS and decision to re-operate on the basis of sole Doppler exam is not justified (although this may well be possible in adult-size renal arteries).
TRAS management
TRAS management consists of three modalities: medical treatment, percutaneous transluminal renal angioplasty (PTRA) with or without stent placement and surgical intervention. The main question to be answered is by which modality to start or how long to treat conservatively before intervening either by angioplasty or surgery?. In some of the literature reports there was no significant difference in the rate of deterioration in renal function, in blood pressure or in the number of anti-hypertensive agents between conservative and interventional treatment [8,22]. If medical treatment is chosen as the first treatment modality, PTA or surgery can however be opted for when conservative measures fail to control hypertension, water retention or if kidney function begins to deteriorate. Cost benefit ratio must be assessed in each individual case because of the risk that PTA or surgical intervention may cause graft loss [23] as for instance in calcified stenosis susceptible to dissection.
Primary PTRA with stent placement is currently the initial treatment of choice for patients with TRAS if the lesion is accessible to such treatment. It is associated with decreased incidence of restenosis (10%) as compared to PTRA alone (16 - 62%) and is less invasive than surgical correction [24]. Immediate technical success of PTA is reported to be over 80% and clinical success is 74 - 87%. Long term clinical success defined as improvement in blood pressure or stabilization or improvement in renal function was reported to be 53 - 70% at one year, with restenosis rate 10 - 33% [23]. One and five year graft survival rate was 91% and 86% respectively in PTRA with stent placement which was better than in surgical intervention [13]. The worst prognosis was noted in patients treated with secondary PTRA after failed surgery or secondary surgery after failed primary PTRA [13]. Complications caused by PTA other than restenosis comprise renal artery dissection, thromboembolism, hematoma and pseudo-aneurism at the puncture site. Reported complication rate is 0 - 10%. Drug eluting stents are justified in TRAS with vessel diameter < 5 mm [25]. PTA is effective and safe therapeutic option also in pediatric population where TRAS is an increasingly recognized cause for post-transplant arterial hypertension [26].
Surgical intervention is rarely needed. It is indicated if primary angioplasty is considered unsuitable because of recent transplant, multiple stenoses, long and narrow stenosis, clinically important kinking of the renal artery, inaccessibility of stenosis or after failure of angioplasty [13]. Surgical options for renal artery reconstruction include resection, revision of the anastomosis, localized endarterectomy, and renal artery patch angioplasty and bypass graft. The success rate and recurrence rate of surgical revascularization has been reported to be similar to PTA with stenting [2,24]. It is however associated with higher rates of morbidity such as graft loss approaching 30% with a recurrence rate of 12% [23,27] and ureteral injury. Reported mortality is up to 5% [1].
Management of TRAS is optimal only if it is patient tailored on a case by case basis. The reason for surgical revascularization with graft in our patient was the inability to distend ostial stenosis of pediatric artery with high pressure PTA attempts. Aorto-ostial lesions either in native vessels or in grafts can be particularly difficult to dilate. High pressure (22 bar) resistance to aorto-coronary ostial stenosis was reported [28]. Failure to dilate renal stenosis with high pressures (20 bar) in pediatric kidney was also described [29]. Other authors report however a successful PTRA of TRAS at the anastomosis in children [30]. The development of subtotal ostial stenosis in our case seems to be a result of several factors such as surgical technique, turbulent blood flow at the anastomosis and eventually a lack of proper ostial growth. Uric acid (534 μM) and LDL cholesterol (2.9 mM) were only moderately elevated, while other parameters described in the literature as possible risk factors for TRAS, such as CMV infection and CaPO4 product (27.4 mg2/dl2) were negative and normal, respectively. The most probable reason for the inability to dilate the ostial part of the artery was surgical. Anastomosis is performed by a running suture which precludes proper dilatation of the vessel. PTRA can even tear the suture and cause dehiscence especially in the early postoperative period. As iliac artery and distal renal artery showed good patency without atherosclerotic plaques or calcifications we decided for bypass graft instead of venous patch. Only 4 claims were reported for surgical aorto-renal bypass in Medicare database of 823 TRAS patients in the period 2000-2005 [2]. We found no report of a successful Dacron bypass graft in an adult patient who received a pediatric kidney and developed TRAS. It was the first such case at our transplant center. It had an excellent short term result. Although at first in 2010 clinically misinterpreted as heart failure symptoms, symptoms the success of revascularization procedure confirmed TRAS as the main cause of patient's problems. This case clearly highlights the problematic definition of the main causative factor for edema and hypertension in combined heart failure and TRAS patients.
The findings suggest that transplanting a small kidney into a heavy patient may be a risk factor for allograft failure [31]. Unfortunately optimal size match in cadaveric kidney transplantation is not always possible. Unmatched size of recipient and donor kidney in our case was probably a partial reason for transplant renal artery stenosis, which was gaining progressively in hemodynamical importance in the years after transplantation. At the time of transplantation donor artery size was not anyhow modified. Pediatric kidney artery, however, grew properly to a normal size except at the site of anastomosis. TRAS management was patient tailored. We opted for conservative therapy as long as kidney function was stable or arterial blood pressure was properly controlled by antihypertensive drugs. When this was no longer possible we opted for PTRA, which unfortunately was pressure resistant. Lastly surgical intervention with placement of dacron graft was mandatory. Immediate clinical success was optimal, while long term success moderate. Whether a different sequence of treatment modalities would bring a better result, remains questionable.
How to assess therapeutic success
The assessment of therapeutic success is problematic as the definition of clinical success is not uniform. There are several parameters to consider in order to assess the efficacy of a certain procedure, i.e. change in arterial lumen or residual stenosis proved by angiography, hemodynamic indices measured by Doppler ultrasound and clinical parameters such as drop in arterial pressure, loss of edema, increase in glomerular filtration and graft survival. All parameters should be evaluated immediately after procedure and several months or years afterwards. Some authors defined clinical success if creatinine dropped for 15%, diastolic pressure for 15% with a number of antihypertensive medications equal to that before PTRA or mean diastolic pressure reduction for more than 10% with a reduced number of antihypertensive drugs [6]. Others considered a drop of creatinine value of more than 0.3 mg/dl (26.5 μM) and/or reduction in the number of antihypertensive medication as clinical success [32]. Improvement was considered significant if there was < 50% of residual stenosis and > 25% improvement in serum creatinine and eGFR levels after 12 weeks post treatment [13]. Various criteria used are the reason for different percent of reported success of a therapeutic procedure.
There was no doubt about an excellent immediate success of the bypass surgery of our patient. Blood pressure dropped, edema vanished and serum creatinine normalized. Antihypertensive drugs were discontinued and he even went through a transient hypotensive period which resolved after the addition of hydrocortisone. Transient hypotension was most probably primarily due to reduced activation of RAAS system after bypass insertion. It is known that angiotensin II stimulates Arap1 protein expression which in turn downregulates AT1 receptor in the renal vasculature [33]. Reduced level of angiotensin II may have caused less vasoconstriction mediated through the AT1 receptors, which resulted in hypotension. Six months after the operation, however, stenosis at the distal end of the graft emerged. Hypertension developed again and creatinine began to rise again. The most probable reason for the stenosis was narrowing by the neointimal neoplasia caused by proliferation and migration of smooth muscle cells and myofibroblasts into the intima [34]. Blood turbulence between renal artery and bypass graft may also have played a role. Secondary or rescue PTRA are reported to have low degree of success [13], which is in accordance with our experience. Secondary PTRA with stent placement was performed, stenosis was lessened, but creatinine fell only slightly and later rose again. The evolution of these events thus shows that a very good short term success may be of relative value when observed on a longer term.
Conclusion
The present case serves as an illustrative example of difficulties encountered when managing patients with demanding TRAS and some novel observations of this transplant complication. First, the starting etiology of the PTA-refractory anastomotic stenosis in this patient was associated with a small diameter of a child donor artery bringing the attention to the special care needed in diagnostic evaluation of delayed graft function when small children vessels are anastomosed into an adult recipient and in later follow-up. Here even high early peak jet velocities above 2 m/s are usually not a sign of resolvable focal stenosis. Second, the stenosis was discovered immediately after the transplantation but a few years later presented a diagnostic challenge due to the relatively stable (although clearly chronically disturbed) ultrasound-Doppler indices and concomitant aortic stenosis giving a clinical picture of decompensated heart failure. The diagnostic uncertainty also drove us to perform a kidney biopsy, which excluded rejection and showed a classical educative picture of juxtaglomerular apparatus hyperplasia. It was not until the successful execution of aorto-renal graft when the diagnosis was clarified due to the massive reduction of body weight with brisk urine diuresis and the discontinuation of a large number of anti-hypertensives that the patient was taking. The early post-operative (bypass) period was marked with a profound orthostatic hypotension which took several weeks to fully spontaneously resolve. Unfortunately later on the patient developed restenosis at the point of insertion of Dacron graft to the renal artery and percutaneous angioplasty was not successful to improve the allograft function. This is in keeping with reports from the literature showing a relatively poor long-term general success of surgical and secondary PTA procedures in this complication. To conclude, although TRAS may be easily discovered with Doppler examination and readily treated with percutaneous radiological interventions, this case shows that there is a subset of patients with refractory stenosis which may give a chronic and long-term disease course with diagnostic challenges and often demanding surgical interventions. They may have a relatively poor outcome and the best surgical approach to such stenoses still remains to be found.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
-
Bruno S, Remuzzi G, Ruggenenti P (2004) Transplant renal artery stenosis. J Am Soc Nephrol 15: 134-141.
-
Hurst FP, Abbott KC, Neff RT, Elster EA, Falta EM, et al. (2009) Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am J Nephrol 30: 459-467.
-
Wong W, Fynn SP, Higgins RM, Walters H, Evans S, et al. (1996) Transplant renal artery stenosis in 77 patients--does it have an immunological cause? Transplantation 61: 215-219.
-
Fervenza FC, Lafayette RA, Alfrey EJ, Petersen J (1998) Renal artery stenosis in kidney transplants. Am J Kidney Dis 31: 142-148.
-
Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, et al. (1996) Post-transplant renal artery stenosis: impact of therapy on long-term kidney function and blood pressure control. J Urol 155: 1860-1864.
-
Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, et al. (2001) Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology 171: 661-662.
-
Audard V, Matignon M, Hemery F, Snanoudj R, Desgranges P, et al. (2006) Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant 6: 95-99.
-
Zupunski A, Buturovic-Ponikvar J (2005) Duplex-Doppler long-term follow-up of renal transplant artery stenosis: case controlled study. Ther Apher Dial 9: 265-269.
-
Bruno S, Ferrari S, Remuzzi G, Ruggenenti P (2003) Doppler ultrasonography in posttransplant renal artery stenosis: a reliable tool for assessing effectiveness of revascularization? Transplantation 76: 147-153.
-
Cook A, Khoury A, Kader K, Hebert D, Navarro O, et al. (2006) Does peak systolic velocity correlate with renal artery stenosis in a pediatric renal transplant population? Pediatr Transplant 10: 608-612.
-
Patel U, Khaw KK, Hughes NC (2003) Doppler ultrasound for detection of renal transplant artery stenosis-threshold peak systolic velocity needs to be higher in a low-risk or surveillance population. Clin Radiol 58: 772-777.
-
de Morais RH, Muglia VF, Mamere AE, Garcia Pisi T, Saber LT, et al. (2003) Duplex Doppler sonography of transplant renal artery stenosis. J Clin Ultrasound 31: 135-141.
-
Ghazanfar A, Tavakoli A, Augustine T, Pararajasingam R, Riad H, et al. (2011) Management of transplant renal artery stenosis and its impact on long-term allograft survival: a single-centre experience. Nephrol Dial Transplant 26: 336-343.
-
Orlic P, Vukas D, Drescik I, Ivancic A, Blecic G, et al. (2003) Vascular complications after 725 kidney transplantations during 3 decades. Transplant Proc 35: 1381-1384.
-
Belzer F, Glass N, Sollinger H (1988) Technical complications after renal transplantation. In: Morris P, Kidney Transplantation: Principles and Practices 3rd (edn) Philadelphia, Saunders 511-532.
-
Pouria S, State OI, Wong W, Hendry BM (1998) CMV infection is associated with transplant renal artery stenosis. QJM 91: 185-189.
-
Fernandez-Najera JE, Beltran S, Aparicio M, Molina P, Gavela E, et al. (2006) Transplant renal artery stenosis: association with acute vascular rejection. Transplant Proc 38: 2404-2405.
-
Etemadi J, Rahbar K, Haghighi AN, Bagheri N, Falaknazi K, et al. (2011) Renal artery stenosis in kidney transplants: assessment of the risk factors. Vasc Health Risk Manag 7: 503-507.
-
Biederman DM, Fischman AM, Titano JJ, Kim E, Patel RS, et al. (2015) Tailoring the endovascular management of transplant renal artery stenosis. Am J Transplant 15: 1039-1049.
-
Willicombe M, Sandhu B, Brookes P, Gedroyc W, Hakim N, et al. (2014) Postanastomotic transplant renal artery stenosis: association with de novo class II donor-specific antibodies. Am J Transplant 14: 133-143.
-
Gunther A, Foss A, Holdaas H, Brabrand K, Hartmann A, et al. (2008) Increased peak systolic velocity in the renal artery of paediatric kidneys transplanted to adult recipients. Nephrol Dial Transplant 23: 4041-4043.
-
Geddes CC, McManus SK, Koteeswaran S, Baxter GM (2008) Long-term outcome of transplant renal artery stenosis managed conservatively or by radiological intervention. Clin Transplant 22: 572-578.
-
Rajan DK, Stavropoulos SW, Shlansky-Goldberg RD (2004) Management of transplant renal artery stenosis. Semin Intervent Radiol 21: 259-269.
-
Henning BF, Kuchlbauer S, Boger CA, Obed A, Farkas S, et al. (2009) Percutaneous transluminal angioplasty as first-line treatment of transplant renal artery stenosis. Clin Nephrol 71: 543-549.
-
Abate MT, Kaur J, Suh H, Darras F, Mani A, et al. (2011) The use of drug-eluting stents in the management of transplant renal artery stenosis. Am J Transplant 11: 2235-2241.
-
Ghirardo G, De Franceschi M, Vidal E, Vidoni A, Ramondo G, et al. (2014) Transplant renal artery stenosis in children: risk factors and outcome after endovascular treatment. Pediatr Nephrol 29: 461-467.
-
Benoit G, Moukarzel M, Hiesse C, Verdelli G, Charpentier B, et al. (1990) Transplant renal artery stenosis: experience and comparative results between surgery and angioplasty. Transpl Int 3: 137-140.
-
Kurbaan AS, Kelly PA, Sigwart U (1997) Cutting balloon angioplasty and stenting for aorto-ostial lesions. Heart 77: 350-352.
-
Haas N A, Ocker V, Knirsch W, Holder M, Lochbuehler H, et al. (2002) Successful management of a resistant renal artery stenosis in a child using a 4 mm cutting balloon catheter. Catheter Cardiovasc Interv 56: 227-231.
-
Spijkerboer AM, Mali WP, Donckerwolcke RA (1992) Renal transplant artery stenosis in children: treatment with percutaneous transluminal angioplasty. Pediatr Radiol 22: 519-521.
-
Amante AJ, Pinon-Barretto SC (2008) The Correlation of Renal Allograft Weight to Metabolic Index Ratios and Glomerular Filtration Rate Among Living-Unrelated Kidney Transplant Patients: A Cross-Sectional Study. Transplant Proc 40: 2313-2318.
-
Hagen G, Wadstrom J, Magnusson M, Magnusson A (2009) Outcome after percutaneous transluminal angioplasty of arterial stenosis in renal transplant patients. Acta Radiol 50: 270-275.
-
Doblinger E, Hocherl K, Mederle K, Kattler V, Walter S, et al. (2012) Angiotensin AT1 receptor-associated protein Arap1 in the kidney vasculature is suppressed by angiotensin II. Am J Physiol Renal Physiol 302: F1313-1324.
-
Owen SC, Li H, Sanders WG, Cheung AK, Terry CM (2010) Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft. J Control Release 146: 23-30.