International Journal of Stem cell Research and Therapy
Heterogeneity and Diversity of Cancer Stem Cells in Glioblastoma
N Sumru Bayin and Dimitris G Placantonakis*
Department of Neurosurgery, Helen L. and Martin S. Kimmel Center for Stem Cell Biology, NYU School of Medicine, USA
*Corresponding author: Dimitris G. Placantonakis, MD, PhD, Department of Neurosurgery, NYU School of Medicine, 530 First Avenue, Skirball 8R, New York, NY 10016, USA, Tel: +1-212-263-2441, Fax: +1-212-263-8042, E-mail: dimitris.placantonakis@nyumc.org
Int J Stem Cell Res Ther, IJSCRT-1-001, (Volume 1, Issue 1), Editorial; ISSN: 2469-570X
Received: August 08, 2014 | Accepted: August 11, 2014 | Published: August 13, 2014
Citation: Bayin NS, Placantonakis DG (2014) Heterogeneity and Diversity of Cancer Stem Cells in Glioblastoma. Int J Stem Cell Res Ther 1:001. 10.23937/2469-570X/1410001
Copyright: ©2014 Bayin NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Glioblastoma (GBM) is the most common and deadly primary brain malignancy, with an annual incidence of over 10,000 new cases in the US and a median survival of 14-16 months [1-3]. The current standard of care includes surgical resection followed by postoperative radiotherapy and treatment with the alkylating agent temozolamide [1]. Sadly, prognosis for GBM patients has increased only incrementally and rather marginally over the past half century [4]. The fact that GBM has lagged behind other solid malignancies in therapeutic advances highlights some of the difficulties that clinicians face in treating GBM: the high degree of brain infiltration, which limits the efficacy of surgical resection; the presence of the blood-brain barrier, which precludes most chemotherapeutic agents from achieving therapeutic concentrations in brain tissue; and the robust radioresistance of GBM tumor cells, which mandates much higher radiation doses than other cancer types.
Two additional biological properties of GBM that have emerged as critical challenges are: its complex cellular hierarchy, which is dominated by stem-like cells (or GBM stem cells - GSCs) [5,6]; and its molecular heterogeneity [7-10]. The concept of cellular hierarchy in tumors and cancer stem cells was first developed in the context of leukemias and later substantiated in solid tumors [11,12].In simple terms, cellular hierarchy in biological systems implies that not all cells are equal in significance or potency. In hierarchical models, relatively undifferentiated stem cells undergo asymmetric cell division to both maintain the stem cell pool (self-renewal; Figure 1Ai) and generate more differentiated and specialized progeny (multipotency; Figure 1Aii). Cancer stem cells in GBM were first described over 10 years ago [5,6]. These stem-like cells self-renew and generate tumor lineages, including endothelium and pericytes of tumor vessels [13-15] (Figure 1Aiii). Importantly, GSCs have increased ability to generate xenograft tumors in the brain of immunosuppressed mice, indicating enhanced tumorigenicity [5]. Finally, GSCs are highly resistant to chemoradiotherapy via intrinsic and microenvironment-mediated mechanisms [16-18] (Figure 1B). These properties suggest a central role for GSCs in tumor growth and recurrence after conventional chemoradiotherapy [19]. Since GSCs play a dominant role in GBM's cellular hierarchy, current therapeutic approaches sparing this population of cells are destined to fail.
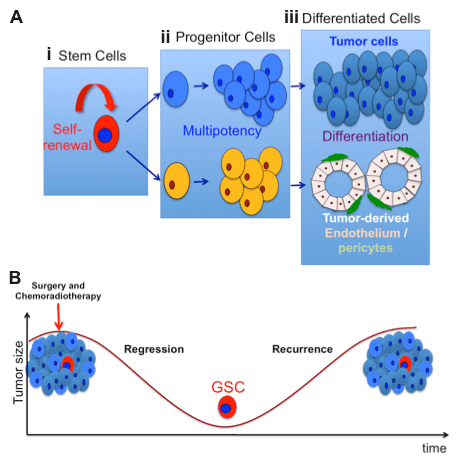 Figure 1: Stem-like cells in GBM and their response to chemoradiotherapy. A. Cancer stem cells in GBM utilize asymmetric
cell division to self-renew (i) and generate progenitor cells (ii) that differentiate to tumor lineages, including tumor endothelium and pericytes (iii). B. Surgical resection and conventional chemoradiotherapy result in cytoreduction and decrease in tumor size. However, GBM stem cells (GSCs) show remarkable resistance to radiation and chemotherapy and generate tumor recurrence.
View Figure 1
Figure 1: Stem-like cells in GBM and their response to chemoradiotherapy. A. Cancer stem cells in GBM utilize asymmetric
cell division to self-renew (i) and generate progenitor cells (ii) that differentiate to tumor lineages, including tumor endothelium and pericytes (iii). B. Surgical resection and conventional chemoradiotherapy result in cytoreduction and decrease in tumor size. However, GBM stem cells (GSCs) show remarkable resistance to radiation and chemotherapy and generate tumor recurrence.
View Figure 1
To complicate matters even further,genomic and transcriptomic analyses of GBM biospecimens have revealed remarkable inter-tumoral heterogeneity at the molecular level [7-10]. Based on the identification of stereotypical gene expression changes or mutations, GBM tumors are classified into 4 broad molecular subtypes: proneural, neural, classical and mesenchymal. The implications of such molecular diversity are clear: different GBM subtypes may show differential responses to the same treatment and future therapeutic approaches will likely have to be tailored to each tumor's unique genetic profile.
The astonishing biological complexity of GBM does not end there, however. Besides the molecular diversity across tumors, there is equally astonishing heterogeneity within any given GBM tumor. At the histologic level, GBM tissue is anything but monotonous: areas of solid tumor are interspersed with necrotic foci surrounded by densely packed tumor cells, a phenomenon called pseudopalisading necrosis. Furthermore, the tumor cells themselves cover a wide spectrum of size, shape and overall morphology. It follows that intra-tumoral heterogeneity may lead to lack of therapeutic response of subsets of tumor cells, even to treatments tailored to specific molecular subtypes. Given this remarkable heterogeneity, how then can we study the biological role and therapeutic relevance of each different cell type within these tumors?
Cancer stem cell research over the past few years has focused on identifying universal markers that identify these cells. However, especially within solid tumors like GBM, where extensive cellular and molecular diversity is the only common denominator amongst the tumor cells, is it meaningful to think that there is only one type of cancer stem cell in any given tumor?. Our laboratory focuses on understanding and dissecting the heterogeneity within the cancer stem cell compartment of GBM with the goal of developing novel meaningful therapies. The rationale and motivation behind our efforts are clear: if these stem-like cells play such an important role in tumor growth and recurrence, and if there is extensive molecular heterogeneity within these tumors, we propose that it is imperative to study and understand whether there is functional and molecular diversity within the stem cell compartment. The question is, then, how do we unravel such heterogeneity?.
The cancer stem cell phenotype in GBM has been linked to several cell surface molecular markers, including CD133, SSEA-1 (CD15) and integrin a6 [5,20,21], as well as signaling cascades that promote self-renewal, such as the Notch and TGFß pathways [22-26]. We therefore asked the simple question: do GBM stem-like cells identified by specific cell surface markers coincide with cells in which signaling pathways relevant to self-renewal are active?. Our findings to date indicate that the overlap between cell surface GSC markers and such signaling pathways is only partial, suggesting the presence of multiple subtypes of GSCs with differential dependence on signaling cascades for their self-renewal and differentiation potential. Interestingly, our data indicate that these GSC subtypes may not only differ at the molecular and developmental level, but also in terms of their basic metabolism [27].
The presence of diverse subtypes of cancer stem cells in GBM is an important concept that will lead to a better understanding of the disease process. We believe that investigating the diversity of cancer stem cells and defining the cellular hierarchy in GBM will lead to development of informed combinatorial therapies not only in GBM, but also in other malignancies that exhibit heterogeneity.
References
-
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. See comment in PubMed Commons below N Engl J Med 352: 987-996.
-
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. See comment in PubMed Commons below N Engl J Med 370: 709-722.
-
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, et al. (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. See comment in PubMed Commons below N Engl J Med 370: 699-708.
-
Netsky MG, August B, Fowler W (1950) The longevity of patients with glioblastoma multiforme. See comment in PubMed Commons below J Neurosurg 7: 261-269.
-
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. (2004) Identification of human brain tumour initiating cells. See comment in PubMed Commons below Nature 432: 396-401.
-
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, et al. (2003) Identification of a cancer stem cell in human brain tumors. See comment in PubMed Commons below Cancer Res 63: 5821-5828.
-
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, et al. (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. See comment in PubMed Commons below Cancer Cell 9: 157-173.
-
Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, et al. (2009) Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. See comment in PubMed Commons below PLoS One 4: e7752.
-
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, et al. (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. See comment in PubMed Commons below Cancer Cell 17: 98-110.
-
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. See comment in PubMed Commons below Nature 455: 1061-1068.
-
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. See comment in PubMed Commons below Nature 367: 645-648.
-
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. See comment in PubMed Commons below Nat Med 3: 730-737.
-
Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, et al. (2010) Glioblastoma stem-like cells give rise to tumour endothelium. See comment in PubMed Commons below Nature 468: 829-833.
-
Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, et al. (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. See comment in PubMed Commons below Nature 468: 824-828.
-
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, et al. (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. See comment in PubMed Commons below Cell 153: 139-152.
-
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. See comment in PubMed Commons below Nature 444: 756-760.
-
Chen J, Li Y, Yu TS, McKay RM, Burns DK, et al. (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. See comment in PubMed Commons below Nature 488: 522-526.
-
Hardee ME1, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, et al. (2012) Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res 72: 4119-4129.
-
Bayin NS, Modrek AS, Placantonakis DG (2014) Glioblastoma stem cells: Molecular characteristics and therapeutic implications. See comment in PubMed Commons below World J Stem Cells 6: 230-238.
-
Son MJ, Woolard K, Nam DH, Lee J, Fine HA (2009) SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. See comment in PubMed Commons below Cell Stem Cell 4: 440-452.
-
Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, et al. (2010) Integrin alpha 6 regulates glioblastoma stem cells. See comment in PubMed Commons below Cell Stem Cell 6: 421-432.
-
Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, et al. (2009) TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. See comment in PubMed Commons below Cancer Cell 15: 315-327.
-
Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, et al. (2009) Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. See comment in PubMed Commons below Cell Stem Cell 5: 504-514.
-
Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, et al. (2010) Notch promotes radioresistance of glioma stem cells. See comment in PubMed Commons below Stem Cells 28: 17-28.
-
Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, et al. (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. See comment in PubMed Commons below Stem Cells 28: 5-16.
-
Chen J, Kesari S, Rooney C, Strack PR, Chen J, et al. (2010) Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. See comment in PubMed Commons below Genes Cancer 1: 822-835.
-
Bayin NS, Dietrich A, Abel T, Chao MV, Song HR, et al. (2014) Glioblastoma Stem Cell Heterogeneity. ISSCR Annual Meeting.





