International Journal of Respiratory and Pulmonary Medicine
Continuous Abstinence Rates at 3, 6, 9 and 12 Months in a Smoking Cessation Unit at the Albacete University HospitalOver 2 Years
Callejas Gonzalez FJ1*, Genoves Crespo M2, Cruz Ruiz J1, Tornero Molina AI1, Esquinas Lopez C3, Bermejo Lopez P4, Garcia Castillo S1, Martinez Garcia AJ1, Rodriguez Montes JA5 and Tarraga Lopez PJ6
1Servicio de Neumologia, Complejo Hospitalario Universitario de Albacete (CHUA), Spain
2Servicio de Cirugia Toracica, Complejo Hospitalario Universitario de Albacete (CHUA), Spain
3Servicio de Neumologia, Hospital Valld'Hebron, Spain
4Grupo SIMD (Sistemas Inteligentes y Mineria de datos) de la Universidad de Castilla-La Mancha (UCLM), Spain
5Servicio de Cirugia General y del Aparato Digestivo, Hospital Universitario La Paz, Spain
6Gerencia de Atencion Integrada, Complejo Hospitalario Universitario de Albacete (CHUA), Spain
*Corresponding author: Francisco Javier Callejas Gonzalez, The Albacete University Hospital Complex, Albacete, Spain, Tel: +34 638225109, Fax: +34 967598002, E-mail: f.javiercallejas@hotmail.com
Int J Respir Pulm Med, IJRPM-2-008, (Volume 2, Issue 1), Research Article; ISSN: 2378-3516
Received: November 26, 2014 | Accepted: December 30, 2014 | Published: January 04, 2015
Citation: Callejas GFJ, Genoves CM, Cruz RJ , Molina AI T, Esquinas LC, et al. (2015) Continuous Abstinence Rates at 3, 6, 9 and 12 Months in a Smoking Cessation Unit at the Albacete University Hospital Over 2 Years. Int J Respir Pulm Med 2:008. 10.23937/2378-3516/1410008
Copyright: ©2015 Callejas GFJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Review the continuous abstinence rates at 3, 6, 9 and 12 months obtained in a Smoking Cessation Unit (SCU) over 2 years.
Methods: Retrospective descriptive analysis of the results obtained from 559 patients attended to in anSCU from January 1st in 2008 to December 31st in 2009, and a subsequent 1-year follow-up.
Results: 838 patients referred 559 attended to and only 278 treated (33%); 55.4% males and 44.6% females; mean age of 46.4 years. The most important comorbidities were psychiatric (36.3%), cardiovascular (dyslipidemia 30.2%, hypertension 19.1%, diabetes 9.4%) and respiratory (chronic obstructive pulmonary disease (COPD), 15.1%; Obstructive Sleep Apnea Syndrome (OSAS), 11.9%; asthma, 7.6%). Treatments included varenicline (VRN), 36.7%, nicotine replacement therapy (NRT), 43.4%, bupropion, 13.3%, and psychological counseling only for 7.9%. Continuous abstinence rates at 3, 6, 9 and 12 months were 48.6% (n=135), 34.2% (n=95), 29.1% (n=81) and 27.7% (n=77), respectively, and failure, abstinence never achieved, was found for 14.7%. Of the 77 successfully treated patients, VRN obtainedthe best success rate (40.3%), followed by NRT (24.7%), psycotherapy (10.4%) and bupropion (10.4%). The 165 patients who relapsed obtained a high relapse rate at 3 months (23.7%), and 113 patients did not relapse.
Conclusion: Treatment success, considered to be continuous abstinence after 1 year, appeared in 27.7% of the sample.
Keywords
Smoking habit, Smoking cessation, Continuous abstinence, Treatment success, Failure, Relapse, Varenicline, Bupropion, NRT, Psycotherapy
Introduction
Smoking is a chronic addictive and relapsing disease that is maintained due to the dependency that nicotine causes. The main clinical manifestations include cardiovascular and respiratory disorders, and tumors appearing in various places [1-3]. In 2014 JAMA published a study that estimated the prevalence of smoking in 187 countries from 1980-2012. This showed that the global percentage of daily smokers has decreased by 25% for men and 42% for women but the absolute number of smokers has increased, probably due to population growth. In 2012 all around the world, smoking prevalence was higher among men than women except in Sweden. Over 50% of men smoke every day in countries like Russia, Indonesia, Armenia and Timor Leste. For women, the prevalence was higher than 25% in Austria, Chile and France, but the country with more smokers proved Greece with more than 30% [4]. According to a published survey in 2009, in Spain the prevalence was 29.9%, 26.2% smoked daily, 3.7% occasionally, 20.4% declared former smoker and 49.7% declared never having smoked [5]. Therefore smoking has a high prevalenceand varies from country to country according to social, economic and cultural criteria, and it is the first cause of death in developed countries [6-9], with about 140 deaths a day. Every year in our country die almost 60.000 smoking and Health expects until 2030 in our planet smoking will kill more than 8 million people every year.
The objective of this article is to present healthcare results, these being continuous abstinence ratesat 3-, 6-, 9-and 12-months follow-up in a specialized Smoking Cessation Unit (SCU)consultation at the Albacete University Hospital Complex over 2 years:1 January 2008 to 31 December 2009.
Patients and Methods
Healthcare resources
In 2003, the Castilla-La Mancha Health Service (SESCAM) developed the “Castilla-La Mancha Smoking Prevention and Treatment Plan, 2003-2010”, whose general objectives included cutting the smoking habit and, therefore, improving the health and quality of life of the community population, the healthcare service treating smoking, cutting smoking among adolescents and creating specialized smoking cessation units in eight hospitals in the area (Albacete, Alcazar de San Juan, Ciudad Real, Cuenca, Guadalajara, Puertollano, Talavera de la Reina and Toledo). The Specialized Smoking Treatment Unit (SSTU) at the Albacete University Hospital Complex (CHUA) is composed of two pneumologists and a female university nursing graduate (UNG) who had previously received specific smoking cessation training. Patients' follow-up comprised a first visit when a general clinical history anda specific clinical history of smoking were created, and a physical examination, which included CO-oximetry, height, weight and blood pressure, was made. Having diagnosed someone as a smoker, a date for smoking cessation and for successive visits were set. Each patient received individual assistance and chose the pharmacological treatment to be used, which was selected according to several parameters (number of cigarettes smoked, nicotine-dependence test, CO-oximetry, patient's comorbidity, contraindications and pharmacological interactions, and patient's preferences, whenever possible. Patients were explained about possible side effects of drugs and instructions for correct use), as well as cognitive-behavioral psychological techniques, contract contingencies, self-analysis of reasons, or cigarette records. The second visit was organized on the day before the date set to start smoking cessation. During this visit, checks were made to see; if smoking had reduced, if it had been programmed; the patient's cigarette record; if pharmacological treatment had begun, if it was being correctly used; if any of the possible side effects were present or not. If pharmacological treatment had not begun, written instructions were handed out to begin treatment on the day smoking cessation was scheduled. During this visit, any signs of withdrawal symptoms were measured for the first time, support documents were handed out, conduct modification techniques were employed, and alternative activities and problem solving were recommended. During successive visits, withdrawal symptoms, CO-oximetry and vital signs were measured, problem solving was provided if necessary, and correct treatment use and any possible side effects were checked. Besides the medical doctor´s experience, the prescription of either drug treatment was based on the clinical characteristics of the patient, the severity of smoking, the personal preferences of each smoker as this may result in better compliance with recommended treatment. The SEPAR (Spanish Society of Respiratory Pathology) guidelines were followed to provide dose and duration of using of prescribed drug treatments. Keep in mind that you can consider the presence of a possible selection bias of patients to treatment groups. This would produce dependence and correlation between factors and treatments may invalidate the statistical inference in relation to the results regarding the effect of treatments. In this sense we must consider, first, that the aim of this study was to analyze the factors that may be related to the results regarding abstinence rate after a follow-up period.
Study description and statistical analysis
A descriptive, retrospective study of the results obtained after analyzing the whole patient sample that came to the SSTUat the Albacete Hospital Complex was conducted from 1 January 2008 to 31 December 2009. Follow-up lasted 1 year. The main parameter used to measure treatment success was the continuous smoking cessation rate over 1 year which, if achieved, was defined as treatment success. Other continuous smoking cessation cut-off points were used:3, 6 and9 months after smoking cessation treatment began. Validation of abstinence was verified by CO-oximetry and the patient's verbal statement. Values were measured with a Micro Smokerlyzer CO-oximeter (Bedfont Scientific; Rochester, UK) by fixing CO concentrations in exhaled air to be under 10ppm. Patients were treated with the psychological and pharmacological combination, using 300mg bupropion, 1mg or 0.5mg varenicline every 12h and/or NRT as 21mg, 14mg and 7mg chewing gum, pillsand/or patches. The study variables were obtained by means of a clinical interview, doing a clinical history review and using a database created with the SPSS software, version 18. First a data quality control was done by identifying any anomalous data in each variable. Then thefrequency and valid percentage of the qualitative variableswere determined, while central trend measures (mean), dispersion measures (interquartile range – IQR) were employed with the quantitative variables.
Results
Sample description
The specialized consultation attended to 838 patients in all, of whom 559 came to the visits (66.7%); 53.7% (n=300) were men and 46.3% (n=259) were women, of whom 281 did not return after the first visit, which left 278 smokers who started treatment (33%). In the studied variables (age, sex and comorbidities) no statistically significant differences were observed among patients referred and patients attending successive visits. Of these 278 who started treatment, 55.4% were male (n=154) and 44.6% were female (n=124), and their mean age was 46.45 years (an age range of18-79 years). The mean age at which smoking commenced was 16.83 years (an age range of 8-years) and the mean number of cigarettes smoked was 27.38cigarettes/day. Patients presented a mean moderate-severe physical dependence (6.13), measured by the Fagerström test, and a high degree of motivation (8.29), according to the Richmond test. The concentrations determined by Co-oximetrygave a mean value of 16.82 with a standard deviation of 9.73. The sample patients reported having made at least 1.43 previous attempts to stop smoking, with a mean duration of 224.49 days of abstinence beforethey made the present smoking cessation attempt. Finally, of the 278 patients (33%) who commenced treatment, 165 (19%) finished it. Figure 1 shows the patients who were sent to the SSTU. The following comorbidities associated with the sample patients were found, and are presented in order of frequency: first, the psychiatric pathology with 36.3% (n=101), of whom 91 patients (32.7%) showed depression/anxiety; second, the groupofcardiovascular comorbidities: dyslipidemia 30.2% (n=84), HBP 19.1% (n=53) and diabetes 9.4% (n=26); lastly, respiratory comorbidity: COPDin 15.1% (n=42), SAHS in 11.9% (n=33) and asthma in 7.6% (n=21).
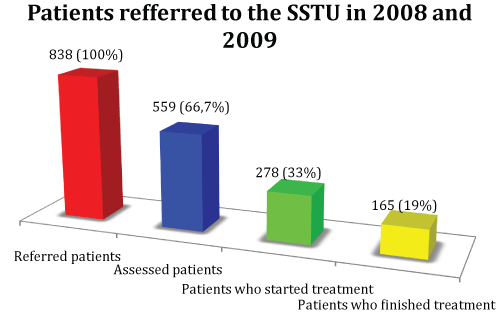
Figure 1: Patients referred to the Specialized Smoking Treatment Unit (SSTU)
of University Hospital Complex in Albacete (CHUA)
View Figure 1
Treatment guidelines
All the patients attended to received personalized pharmacological treatment and/or psychotherapy. Of the 278 patients, 36.7% (n=102) were treated with varenicline (95 of them with 1 mg/12 h; 4 patients with 0.5mg/12 h; 2 with VRN and patches and 1 on varenicline and nicotine gum); in 43.4% (n=117) of the cases NRT was administered to, with bupropion prescribed to 37 patients (13.3%), and only psychological counseling was given to 22 cases (7.9%). Table 1 offers the frequencies and percentages of the treatments prescribed to the patients in the study sample.
![]()
Table 1: Frequencies and percentages of the treatments prescribed to the
sample of patients
View Table 1
Treatment success
The frequencies and percentages of the abstinence rates at 3, 6, 9 and 12 months were collected (Table 2). Of the 278 treated patients, 27.7% (n=77) were successfully treated, as the table shows Table 3 reflects the success frequency ofthe whole sample (n=278) for each prescribed treatment. Of the 77 patients who achieved continuous abstinence after 1 year, the highest success percentage was obtained for those patients treated with varenicline (40.3%, n=31), followed by NRT using 14 mg patches every 24 h for 42 days and the 7 mg patches for another 14-day period (24.7%, n=19), and then by psychotherapy (10.4%, n=8) and bupropion (10.4%, n=8). In overall study sample, success in smoking cessation was 18.9% in patients who developed respiratory comorbidity, 3.2% in those suffering cardiac pathology and 10.4% in patients with psychiatric comorbidity. No success was showed in 100% of patients who associated concomitant cardiovascular and respiratory comorbidity.
![]()
Table 2: Continuous abstinence rates at 3, 6, 9 and 12 months at the SSTU of
CHU in Albacete
View Table 2
![]()
Table 3: Success according to the treatments used in the SSTU of CHU in
Albacete
View Table 3
Relapses, failure and reasons to drop out
Table 4 shows that 113 of the 278 patients did not relapse. The third month was when more relapses occurred (n=66, 23.7%). The reasons for dropping out of the study sample were side effects in 13 patients (4.7%), the patient's own decision in 77 cases (27.7%) and other causes in 14 patients (5%).
![]()
Table 4: Relapses in patients treated at the SSTU of CHU in Albacete (expressed
as frequency and percentage)
View Table 4
Discussion
Our sample's epidemiological data
In Spain, the mean age when smoking commences is around 13.4 years [1,2,5,6,10]. In our study sample, this mean age was 16.83 years (an age range of8-36 years). Overall smoking prevalence has lowered over the years, although this reduction is basically attributed to men since smoking prevalence has actually increased among women [10]. Our study showed a slight male predominance (55.4% males vs. 44.6% females), which confirmed today's trend. Heavier smokers are usually aged between 45-64 years [10,11], so it is logical that the mean age of our patients was 46.45 years.Smoking is a pathology that causes serious comorbidities, such as cardiovascular and respiratory disorders, and tumors in several places [1,2,6], among others. The most outstanding pathologies in our study sample were of the psychiatric and respiratory kind, as well as cardiovascular risk factors.
Treatment employed
Of all the patients in this study, 100% received personalized treatment, psychological cognitive-behavioral therapy, and they were all offered pharmacological treatment. It is worth stressing that 7.9% of the patients did not receive pharmacological treatment, but received only psychological counseling. All the published meta-analyses coincide in pointing out that smoking treatment efficacy does not depend on the format it is offered in [9,12,13]. The indication of one format or another depends on the characteristics of the population being attended to and the therapeutic team's working methodology. According to the characteristics of the consultation, space and personnel, our group used only an individual format. Prescribing one pharmacological treatment or another is based on the patient's clinical characteristics, the degree and severity of smoking, and each smoker's personal preferences [9,14]. NRT was the most widely used therapy, regardless of chewing gum or patches, or it being combined with varenicline, as 42.2% of the sample accepted it, followed by varenicline (36.7%), and finally, by bupropion (13.3%). In all cases, taking the smoker's preferences into account before suggesting any therapy type was recommended because this could help achieve better compliance to recommended treatments [12].
Dropout rates
We should stress the high dropout rate that we recorded while the 2-year check-ups were underway; only 66.7% of the patients who were initially sent to the consultation continued coming. Of those who came, 50.3% also dropped out. Therefore, only 33% of the total initial study sample started treatment. Perhaps several factors could explain this fact;many of the patients who were sent to the consultation did not voluntarily apply to do so, but were referred by their specialist. During the first consultation, to a lesser extent, patients sometimes explained the difficulties they had to come to the consultation because opening times were incompatible; they had problems getting there, among others. There were even patients who stated that treatment was expensive. This high dropout rate made us consider two needs: making available adequate knowledge of the criteria to refer patients to the specialized consultation; suitable training in this field of the healthcare personnel who leads the SSTU correctly assessing and informing smoking patients in orderto optimize resources and to improve the general healthcare offered to the smoking population in the province of Albacete.
Overall success, relapses and treatment used
One of the difficulties of conducting studies into treating smoking is assessing its efficacy as exactly as possible. The results are always estimated based on what patients state and on confirmation made by biological methods, among which the CO test is the most widely used [11]. Another difficulty lies in having to measure efficacy and not overestimating the obtained results. Hence abstinence tends to be defined as the results obtained from intention to treat; that is, the confirmed success percentages of all patients who started treatment. Our study employed the compliance of both criteria as a success criterion. Another relevant aspect to bear in mind is to consider continuous abstinence based on the patient's and/or family relation's self-declaration, and measuring CO-oximetry. The self-declaration procedure is widely used in studies into smoking [15-19]. Although some authors have confirmed a percentage (which is quite often a considerable figure) of subjects who lie about their smoking habits while visiting their doctor [20], a sufficiently high percentage of reliable answers are given by subjects, to the extent that the test to determine the CO exhaled in air could be done away with, and not even be used to verify abstinence [21]. Some of the variables associated with the abstinence time in this study were having received medical treatment to stop smoking or not, treatment type (NRT, varenicline or bupropion) and the number of cigarettes smoked. Use of treatment combined with smoking cessation counseling multiplies the possibilities of success by 1.7, if compared to receiving only psychological counseling, and [9] by 1.4 as opposed to receiving only pharmacological treatment. All the patients in our sample received cognitive-behavioral psychological therapy and were also offered a pharmacological treatment. Of the whole sample, 7.9% received only psychological counseling, with a success rate of only 10.4% as opposed to success rates of up to 40.3% for those who received a combined therapy with VRN, either in association with other treatments or not, as well as psychological counseling. The results obtained for abstinence from smoking over 1 year can vary in specialized smoking units, with ranges covering 14.9-54.9% depending on the place applied and treatment type employed [15,22-25]. The literature includes many studies that have compared abstinence rates for the drugs we used in our study and also for placeboes. It is very important to mention that the aim of our study was not to compare the results obtained with other published original investigations due to it was a small descriptive sample. Finally, the Table 5 shows the results obtained in different Spanish specialized units, including those we report herein. Basically, if we bear in mind the major differences among them in terms of design and ways of acting, in comparison to the results of these units, the results of the Albacete unit were not that different since the overall continuous abstinence rates obtained were 48.6% at 3 months, 34.2% at 6 months, 29.1% at 9 months and 27.7% at 1 year. The causes of the low success rates obtained for smoking cessation could be related with the fact that the sample included heavy smokers who had been smoking for years and who had significant associated comorbidities. A high post-treatment relapse rate has also been reported in other studies [15]. It is known that the long-term probability of abstinence is related with persisting with abstinence since treatment begins and throughout follow-up [15-17]. In our study sample, abstinence at 1 year was 27.7%, which falls, therefore, within the range of percentages reflected in other studies, and most relapses occurbasically during the first 3 months (23.7%), which coincides with other series [22,29]. To conclude, we the authors believe that despite the fact that much work still needs to be done to obtain the best smoking cessation results, above all in primary and secondary prevention health care, the continuous abstinence rates in our specialized consultation, and the differences found with other units, are in line with similar published results.
![]()
Table 5: Table comparing the continuous abstinence rates at 3, 6, 9 and 12
months in various Spanish studies and our consultation
View Table 5
References
-
The World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
-
WHO. International Classification of Diseases (ICD). Disponible en.
-
deGrandaOrive JI E (2007) tabaquismocomoenfermedadadictivacronica. En: Jimenez-Ruiz CA y Fagerström KO (Ed). Tratado de Tabaquismo. (2nd Edn) Madrid. Ergon: 99-119.
-
MarieNg, Freeman MK, Fleming TD, Robinson M, BA, et al. (2014) Smoking Prevalence and CigaretteConsumption in 187 Countries, 1980-2012. JAMA 311:183-192.
-
Instituto Nacional de Estadistica y Ministerio de Sanidad, Politica Social e Igualdad (2009) EncuestaEuropea de Saluden Espana.
-
Jimenez-Ruiz CA, Fagerström KO (2012) El tabaquismocomoenfermedadcronica, Vision global. Tratado de Tabaquismo. 3a Edicion, Madrid, Aula Medica3-10.
-
Fagerström K, Balfour DJ (2006) Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert OpinInvestig Drugs 15:107-116.
-
Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, et al. (2008) Treating Tobacco use and dependence: 2008 update. Clinical practice guideline, Rockville MD: US. Deparment of Health and Human Service. Traduccion al espanol: Guia de TratamientodelTabaquismo. Jimenez-Ruiz CA, Jaen CR (coordinadores de la traduccion). Sociedad Espanola de Neumologia y CirugiaToracica. SEPAR; mayo 2010.
-
Banegas JR, Diez L, Banuelos B, Gonzalez J, Martin-Moreno JM, et al. (2010) La mortalidadatribuible al consumo de tabacoenEspanaen 2006. Med Clin (Barc)
-
Ministerio de Sanidad y Consumo. Instituto Nacional de Estadistica. Encuesta Nacional de Salud de Espanaanos 1987, 1993, 1995, 1997, 2001, 2003 y 2006. Madrid.
-
Gariti P, Alterman AI, Ehrman R, Mulvaney FD, O'Brien CP (2002) Detecting smoking following smoking cessation treatment. Drug Alcohol Depend 65: 191-196.
-
Jimenez-Ruiz CA, de GrandaOrive JI, Solano Reina S, Carrion F, Romero Palacios P, et al. (2003) Recomendaciones para el tratamiento del tabaquismo. Arch Bronconeumol 39: 514-523.
-
Stead LF, Lancaster T (2005) Group behavior therapy programs for smoking cessation. Cochrane Database Syst Rev 18: CD001007.
-
Jimenez-Ruiz CA, Riesco Miranda JA, Ramos Pinedo A, Barrueco Ferrero M, Solano Reina S, et al. (2008) Recomendaciones para el tratamientofarmacologico del tabaquismo. Propuesta de financiacion. Arch Bronconeumo l44: 213-219.
-
Guallar-Castillon P, LafuenteUrdinguio P, Garteizaurrekoa Dublang P, Sainz Martinez O, Diez Azcarate JI, et al. (2003) Probabilidad de exitoen el abandono del tabacoen el curso de dos intervencionessencillas para dejar de fumar. Rev Esp Salud Pública 77: 117-124.
-
Barrueco M, Torrecilla M, Maderuelo JA, Jimenez Ruiz C, Hernandez Mezquita MA, et al. (2001) Valor predictivo de la abstinenciatabaquica a los 2 meses de tratamiento. Med Clin (Barc) 116: 246-250.
-
Nerin I, Novella P, Crucelaegui A, Beamonte A, Sobradiel N, et al. (2004) Factorespredictivos de exito a los 6 mesesenfumadorestratadosenunaunidad de tabaquismo. Arch Bronconeumol 40: 558-562.
-
Marcos T, Godas T, Corominas J (2004) Tratamiento de sustitucion de nicotinafrente a reduccionprogresivaen la deshabituaciontabaquica. Med Clin (Barc) 123:127-130.
-
Sampablo Lauro I, Carreras JM, Lores L, Quesada M, Coll F, et al. (2002) Smoking cessation and bupropion: anxiety and depression as predictors of therapeutic efficacy. Arch Bronconeumol 38: 351-355.
-
Lores Obradors L, Monso Molas E, Rosell Gratacos A, Badorrey I, Sampablo Lauro I (1999) Do patients lie about smoking during follow-up in the respiratory medicine clinic?. Arch Bronconeumol 35: 219-222.
-
Barrueco M, Jimenez Ruiz C, Palomo L, Torrecilla M, Romero P, et al. (2005) Veracity of smokers' reports of abstinence at smoking cessation clinics. Arch Bronconeumol 41: 135-140.
-
Pascual-Lledo JF, De la Cruz-Amoros E, Bustamante-Navarro R, Buades-Sanchez MR, Contreras-Santos C, et al. (2006) Smoking cessation after 12 months follow-up at a smoking cessation unit. Med Clin (Barc) 126: 601-606.
-
Barrueco Ferrero M, Jimenez Ruiz C, Palomo Cobos L, Torrecilla Garcia M, Romero Palacios P, et al. (2004) Continuous and momentous tobacco abstinence with pharmacologic therapy in clinical practice. Med Clin (Barc) 123: 652-656.
-
Jimenez Ruiz CA, Mayayo Ulibarri M, Cicero Guerrero A, Amor Besada N, Ruiz Martin JJ, et al. (2009) Care results in a specialist stop-smoking unit. Arch Bronconeumol 45: 540-544.
-
Russell MA, Stapleton JA, Feyerabend C, Wiseman SM, Gustavsson G, et al. (1993) Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. BMJ 306: 1308-1312.
-
Neumann T, Rasmussen M, Ghith N, Heitmann BL, Tonnesen H (2012) The Gold Standard Programme: smoking cessation interventions for disadvantaged smokers are effective in a real-life setting. Tobacco Control 2011-050194.
-
Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A (2012) Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. EurRespir J 40: 548-554.
-
Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, et al. (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337: 1195-1202.
-
Bauld L, Judge K, Platt S (2007) Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tob Control 16: 400-404.





