International Journal of Pathology and Clinical Research
A Case of Dedifferentiated Leiomyosarcoma of the Uterus
Kanae Nosaka1,2, Hiroaki Komatsu3, Tetsuro Oishi3, Yasushi Horie2, Tasuku Harada3 and Yoshihisa Umekita1*
1Division of Organ Pathology, Department of Pathology, Tottori University, Japan
2Division of Anatomic Pathology, Tottori University Hospital, Japan
3Division of Reproductive - Perinatal Medicine and Gynecologic Oncology, Department of surgery, Tottori University, Japan
*Corresponding author:
Yoshihisa Umekita, MD, PhD, Division of Organ Pathology, Department of Pathology, Faculty of Medicine, Tottori University, 86 Nishicho, Yonago, Tottori 683-8503, Japan, E-mail: yume@med.tottori-u.ac.jp
Int J Pathol Clin Res, IJPCR-2-049, (Volume 2, Issue 4), Case Report; ISSN: 2469-5807
Received: October 26, 2016 | Accepted: November 25, 2016 | Published: November 28, 2016
Citation: Nosaka K, Komatsu H, Oishi T, Horie Y, Harada T, et al. (2016) A Case of Dedifferentiated Leiomyosarcoma of the Uterus. Int J Pathol Clin Res 2:049. 10.23937/2469-5807/1510049
Copyright: © 2016 Nosaka K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Dedifferentiation of leiomyosarcoma is a rare phenomenon that associates pleomorphic histology and loss of smooth muscle differentiation. Although the leiomyosarcoma is well known sarcoma in the uterus, and the dedifferentiated leiomyosarcoma is well recognized in the soft parts, there are only a few reports of dedifferentiation of leiomyosarcoma in the uterus. Herein we present a case of dedifferentiated uterine leiomyosarcoma and discuss its relation to the undifferentiated uterine sarcoma.
Keywords
Uterine leiomyosarcoma, Dedifferentiation, Undifferentiated uterine sarcoma, CD10
Abbreviations
MRI: Magnetic Resonance Imaging; SMA: Smooth Muscle Actin; CEA: Carcinoembryonic Antigen; ER: Estrogen Receptor; PgR: Progesterone Receptor
Introduction
Leiomyosarcoma is relatively rare disease of the uterine corpus accounting for 1-2% of all malignancies according to the World Health Organization (WHO) classification of tumours of female reproductive organs [1]. On the other hand, leiomyosarcoma is also important sarcoma of extra-uterine site and actually major sarcoma in the retroperitoneal and the pelvic areas [2]. Dedifferentiation is a rare phenomenon of soft tissue sarcomas including leiomyosarcoma and is extremely rare among uterine sarcomas [2-8]. Dedifferentiated tumors often exhibit pleomorphic high-grade histology resembling pleomorphic undifferentiated soft tissue sarcoma (USTS) that has been known as pleomorphic malignant fibrous histiocytoma (MFH) in the past [5,6,9,10]. Dedifferentiation is also demonstrated by loss of lineage-specific marker expression by immunohistochemistry. The uterine high grade sarcomas with unknown lineage are called as undifferentiated uterine sarcomas (UUS) and often demonstrate similar morphology with USTS. We present a case of uterine leiomyosarcoma with dedifferentiation and it’s highly pleomorphic dedifferentiated area showed reduced expression of smooth muscle markers.
Case Report
Clinical history
A 63-year-old multiparous female presented with bloody vaginal discharge lasting for 2 months. She had no particular medical history except for the uterine leioimyoma discovered 13 years before. Her cervical cytology was negative while endometrial cytology detected atypical cells. There was no elevation of tumor markers (CEA and CA19-9) in her serum. Hysteroscopy revealed a smooth-surfaced massive tumor occupying the uterine cavity. Abdominal MRI revealed 7.7 × 9.4 × 12.4 cm of round mass in the anterior uterine corpus. The lesion showed heterogeneous high intensity on T2 weighed image and was unevenly enhanced on dynamic contrast study. On the histological examination by biopsy, the tumor tissue was composed of spindle shaped atypical cells with blunt-ended nuclei and eosinophilic cytoplasm, arranged in irregular fascicular pattern intermingled with necrotic area. These tumor cells were positive for α-smooth muscle actin (α-SMA), calponin, desmin, estrogen receptor (ER) and progesterone receptor (PgR) but negative for CD10 by immunohistochemical (IHC) examination. MIB-1 index was around 50% and the tumor was diagnosed as leiomyosarcoma. Thereafter she was transferred to our hospital for further investigation and treatment, and second biopsy was performed. The tissue contained highly atypical cells including bizarre cells, multinucleated neoplastic giant cells and numerous mitotic figures against heavily necrotic backgrounds, rather resembled pleomorphic USTS or UUS than ordinary leiomyosarcoma observed in the previous biopsy. CT examination revealed multiple small nodules in bilateral lungs which were diagnosed as metastases. She underwent simple hysterectomy with bilateral salpingo-oophorectomy and has been receiving adjuvant chemotherapy.
Gross findings
Resected uterus was longitudinally cut in the anterior wall and roughly lobulated polypoid tumor was exposed. After fixation in 10% formalin, the tumor was 14 × 10 × 10 cm in maximum dimension and felt elastic soft (Figure 1a). Stem of the tumor was attached to the uterine fundus. On sagittal slice, proximal part of the tumor looked solid with whitish cut surface containing necrotic foci and hemorrhages (Figure 1b). The whitish area lacked whorl pattern which is characteristic of leiomyoma. About a half of mass distal to the fundus was tan tinted, heavily necrotic and hemorrhagic. The posterior and the lateral uterine wall contained usual leiomyomas. No remarkable change was found in the uterine cervix and uterine appendages.
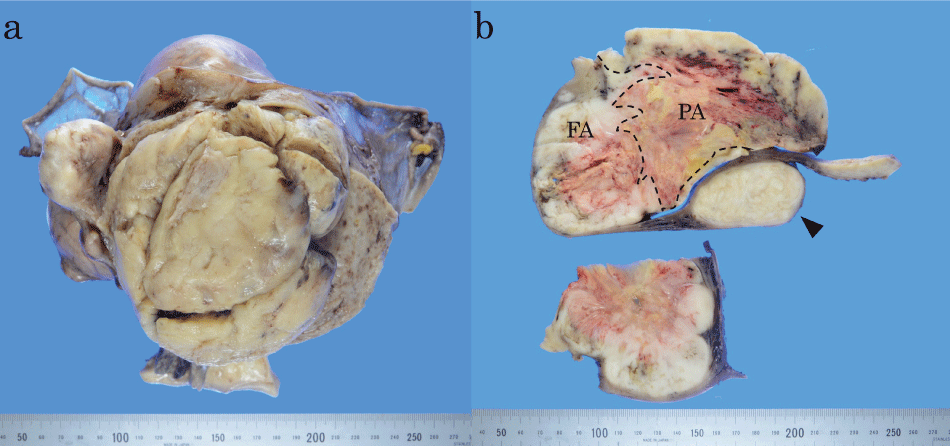
.
Figure 1: Gross findings of the dedifferentiated leiomyosarcoma.
a) Resected uterus was cut longitudinally in the anterior wall and the tumor was exposed. The polypoid tumor was lobulated and attached to the uterine fundus; b) Sagittal slice of the tumor (above) and horizontal slice of right side (below). On the sagittal slice, the fascicular area (FA) is to the left and the pleomorphic area (PA) to the right. Dashed line denotes assumed borderline between these areas; borderline in central necrotic area was postulated from morphology of tumor cells with coagulation necrosis. A leiomyoma was seen in the posterior uterine wall (arrowhead).
View Figure 1
Histological findings
Entire sagittal slice was cut into sections for histological assessment. Microscopically, the proximal part of the tumor (whitish area) was composed of atypical spindle cells with brunt-ended elongated nucleus and eosinophilic cytoplasm, demonstrating high mitotic activity, as many as 50 mitoses per 10 high power fields on average (Figure 2a). There were occasional multinuclear giant cells and mild anisokaryosis, but tumor cells were arranged in the fascicular manner and each cell sustained some resemblance to smooth muscle cells, indicating well-differentiated state. This fascicular part contained occasional geographical necrosis. On the other hand, lower part of tumor (tan tinted area) consisted of cells with marked nuclear atypia, often bizarre in shape. Polygonal large cells rich in pale eosinophilic to amphophilic cytoplasm and occasional multinucleated huge cells as well as broad spindle cells were arranged in irregular fascicle like pleomorphic USTS (Figure 2b and Figure 2c). Occasional storiform arrangement was observed. Some of this pleomorphic area contained numerous hyalinized small vessels. There were heavy necrosis and hemorrhagic foci. An abrupt transition between the fascicular and the pleomorphic area was observed (Figure 2d). No heterologous elements were detected.
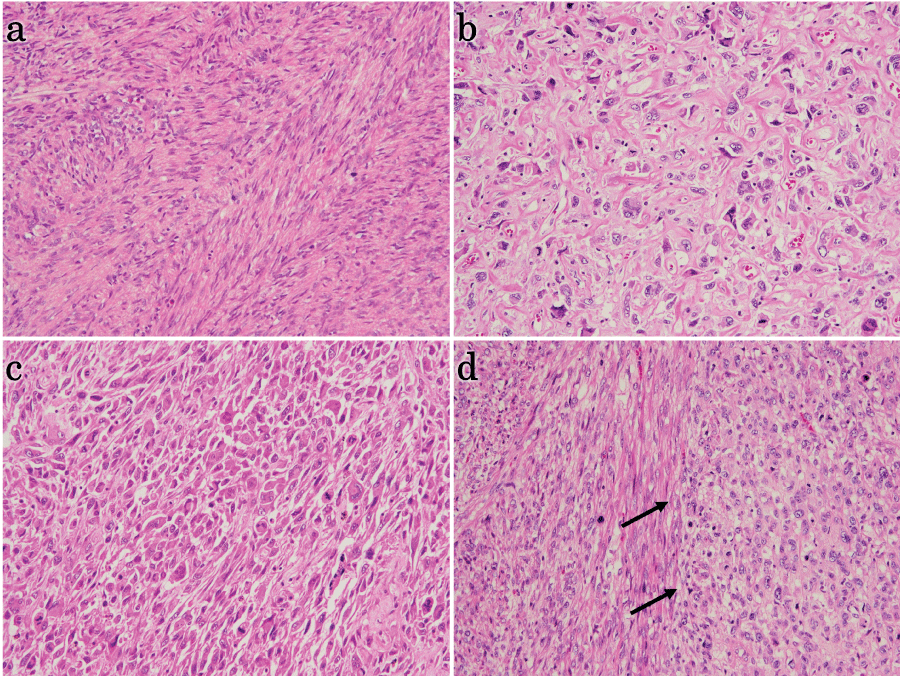
.
Figure 2: Histological finding of the dedifferentiated leiomyosarcoma in hematoxylin-eosin section (Original magnification: 200x [a-c] and 100x [d]).
a) Fascicular area. Tumor cells were spindle with eosinophilic cytoplasm, arranged in fascicular pattern. There were scattered numerous mitotic figures including atypical ones; b) Pleomorphic area. Tumor cells were highly pleomorphic with abundant bizarre cells. Some area was rich in hyalinized stroma and contained many hyalinized vessels as well; c) Polygonal or epithelioid tumor cells were also seen in some area; d) Interface of each area is shown (arrows). The fascicular area is to the left side and the pleomorphic area composed of eosinophilic epithelioid cells to the right. Transition between these components was abrupt.
View Figure 2
Immunohistochemical examination
A tissue section containing both fascicular and pleomorphic area was selected for IHC examinations. Formalin-fixed paraffin-embedded tissues were 4-μm-cut and placed onto silane coated grass slides. Deparaffinized and pretreated tissue sections were processed by automatic immunostaining instrument, Histostainer36A (Nichirei bioscience Inc., Tokyo, Japan) according to the manufacturer's instructions. Results of IHC study are summarized in table 1and shown in figure 3. IHC data in normal myometrium contained in the section is also shown in table 1 as either positive or negative controls. Fascicular area of tumor was positive for several smooth muscle markers (α-SMA, desmin, h-caldesmon, calponin), whereas pleomorphic area was diffusely positive for α-SMA and only focally positive for calponin. Regarding hormone receptors, fascicular area was positive only for ER in contrast to the first biopsy specimen's results. Pleomorphic area was negative either for ER or for PgR. Pleomorphic area was diffusely positive for CD10 and p16, both of which were absent in fascicular area. p53 was positive in both areas, whereas cytokeratin AE1/3, S-100, c-kit and CD34 were negative in both areas. From these findings, we finally concluded that the lower pleomorphic part of this tumor represents dedifferentiation from leiomyosarcoma.
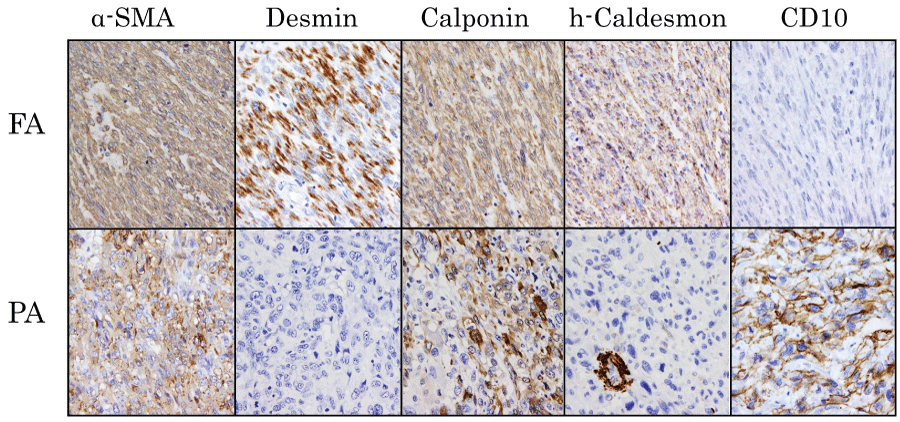
.
Figure 3: Immunohistochemical examination of fascicular and pleomorphic area. (Original magnification: 400x).
Results in the fascicular area (FA) are shown in the upper column and those in the pleomorphic area (PA) are shown in the lower one. In the pleomorphic area, cells showing positivity for h-caldesmon in the figure are vascular smooth muscle cells.
View Figure 3
![]()
Table 1: Results of immunohistochemical examination.
View Table 1
Discussion
Among soft tissue tumors, dedifferentiation is infrequent but well-known phenomenon such as dedifferentiated liposarcoma and pleomorphic rhabdomyosarcoma [2,11]. However, the dedifferentiation of leiomyosarcomas has only been described as occasional phenomenon, without particular designation as a subtype in WHO classification of tumours soft tissues and bone [2] and in Armed Forces Institute of Pathology (AFIP) atlas of tumors of the Soft Tissues [11]. Whereas, the dedifferentiation of uterine leiomyosarcomas have not even been mentioned in WHO classification of tumours of female reproductive organs [1]. To our knowledge, there are only five case reports of uterine leiomyosarcoma with dedifferentiation (Table 2) [4,7,8]. Dedifferentiated leiomyosarcomas are composed of pleomorphic atypical cells with high mitotic rate and heavy necrosis, and often exhibit abrupt transition from well differentiated areas. These dedifferentiated areas often resemble pleomorphic USTS [5,6,9,10]. Dedifferentiation is usually proved by complete loss of lineage-specific markers in immunohistochemistry, and partial preservation of smooth muscle markers like in our case is distinguished as pleomorphic leiomyosarcoma by some authors [6,10].
![]()
Table 2: Dedifferentiated leiomyosarcoma of the uterus.
View Table 2
Dedifferentiated and pleomorphic leiomyosarcomas of soft parts have been shown to be highly aggressive tumors presenting 50% to 65.2% of mortality and 89% of incidence of metastasis [6,10]. It has also been reported that the loss of myogenic differentiation in leiomyosarcoma could be a significant prognostic factor, accounting for an aggressiveness of these tumors [9].
Oda, et al. reported that pleomorphic leiomyosarcoma accounts for 8.6% of all leiomyosarcoma [10], whereas Nicolas, et al. implied higher incidence, for such tumor with tiny differentiated area might have been misdiagnosed as pleomorphic MFH [6]. Just like Demicco, et al. assumed that many of USTSs would be actually dedifferentiated leiomyosarcomas [9], those tumors reported as uterine MFH or UUS might have been dedifferentiated leiomyosarcoma. Indeed, the pleomorphic area of the present case was hardly distinguishable from pleomorphic UUS, since UUS can be positive for CD10 and focally positive for myogenic markers [1].
UUS has now been considered to arise from heterogeneous precursors and to be a diagnosis of exclusion [12] like USTS in soft tissues, requiring extensive histopathological examination for final diagnosis. UUS is known to display transition from endometrial stromal tumors of lower grade, suggesting some UUS are dedifferentiated endometrial stromal tumors, which is also supported by experiments in genetic level [13]. Kurihara, et al. demonstrated that transcripts of JAZF1-JJAZ, a fusion gene detected in 50% of low-grade endometrial stromal sarcoma (ESS), was also detected in 33% of UUS with nuclear uniformity, whereas this transcript was negative in all UUS with nuclear pleomorphism [13]. This finding implies that UUS of pleomorphic type has an origin other than ESS. On the other hand, Rawish, et al. [7] documented an undifferentiated sarcoma arising from uterine leiomyosarcoma, suggesting that UUS could be derived from leiomyosarcoma. As for our case, pleomorphic part was obviously leiomyosarcoma-derived, because neither ESS nor other well differentiated components were observed. These findings imply that significant cases of uterine dedifferentiated leiomyosarcoma might have been diagnosed as UUS, due to insufficient examination or overlooking for differentiated area. Extensive examination is prerequisite for the diagnosis of uterine dedifferentiated leiomyosarcoma.
In the present case, CD10 was diffusely positive in pleomorphic area despite of complete negativity in fascicular area. CD10 is known to be constantly expressed in some malignant tumor such as renal cell carcinoma and ESS [12,13]. Especially among uterine sarcomas, CD10 has been regarded as a useful marker for distinguishing ESS from leiomyosarcoma [14]. However, Mikami, et al. [15] pointed out that some high-grade uterine leiomyosarcomas could express this marker, and postulated that CD10 expression might imply one of the characteristics of Müllerian system-derived neoplastic mesenchymal cells. In turn, Chu, et al. [16] found CD10 expression in 18% of pleomorphic, high-grade spindle cell sarcomas including extra-uterine cases. Therefore, it may be rather generalized event that high-grade sarcoma shows CD10 expression than restricted phenomenon to Müllerian derived tumors.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
-
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC Press, 139-140.
-
Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (2013) WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press, 111-113.
-
Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M (1986) Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 57: 2077-2088.
-
Fukuda T, Ohnishi Y (1991) Histological and immunohistochemical observations of dedifferentiated leiomyosarcoma of the uterus. Acta Pathol Jpn 41: 466-472.
-
Chen E, O'Connell F, Fletcher CD (2011) Dedifferentiated leiomyosarcoma: clinicopathological analysis of 18 cases. Histopathology 59: 1135-1143.
-
Nicolas MM, Tamboli P, Gomez JA, Czerniak BA (2010) Pleomorphic and dedifferentiated leiomyosarcoma: clinicopathologic and immunohistochemical study of 41 cases. Hum Pathol 41: 663-671.
-
Rawish KR, Fadare O (2012) Dedifferentiated leiomyosarcoma of the uterus with heterologous elements: a potential diagnostic pitfall. Case Rep Obstet Gynecol 2012: 534634.
-
Iihara K, Hirano K, Fujioka Y, Sakamoto A (2007) Leiomyosarcoma with dedifferentiation in a premenopausal patient discovered after uterine artery embolization. Pathol Int 57: 681-687.
-
Demicco EG, Boland GM, Brewer Savannah KJ, Lusby K, Young ED, et al. (2015) Progressive loss of myogenic differentiation in leiomyosarcoma has prognostic value. Histopathology 66: 627-638.
-
Oda Y, Miyajima K, Kawaguchi K, Tamiya S, Oshiro Y, et al. (2001) Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol 25: 1030-1038.
-
Miettinen M, Fetsch JF, Antonescu CR, Folpe AL, Wakely PE (2014) Tumors of the soft tissues: AFIP atlas of tumor pathology series 4, fascicle 20: Maryland, ARP press, 523.
-
Ali RH, Rouzbahman M (2015) Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. J Clin Pathol 68: 325-332.
-
Kurihara S, Oda Y, Ohishi Y, Iwasa A, Takahira T, et al. (2008) Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J SurgPathol 32: 1228-1238.
-
Chu PG, Arber DA, Weiss LM, Chang KL (2001) Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol 14: 465-471.
-
Mikami Y, Hata S, Kiyokawa T, Manabe T (2002) Expression of CD10 in malignant müllerian mixed tumors and adenosarcomas: an immunohistochemical study. Mod Pathol 15: 923-930.
-
Chu P, Arber DA (2000) Paraffin-section detection of CD10 in 505 nonhematopoietic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol 113: 374-382.





