International Journal of Oral and Dental Health
The Potential of Human Dental Pulp Stem Cell from Deciduous Teeth in Bone Regeneration: An Experimental Study in Rabbits
Gustavo C Belmonte1, Leandro A Holgado1, Roger William de Labio2, Patrícia Sammarco Rosa3, Rosimeire Segato2, Josianne T Fukasawa2, Bruno Solano de Freitas Souza4,5, Ricardo Ribeiro dos Santos4, Oswaldo Baffa6, Spencer L M Payão1,2 and Angela Kinoshita1,6*
1Pró-Reitoria de Pesquisa e Pós Graduação, Universidade do Sagrado Coração, Rua Irmã Arminda 10-50, Bauru, São Paulo, Brazil
2Faculdade de Medicina de Marília, Marília, Av. Monte Carmelo, 800, Marília, São Paulo, Brazil
3Instituto Lauro Souza Lima, Rodovia Cte Joao Ribeiro de Barros, S/N/Km 225 226 Bauru, São Paulo, Brazil
4Hospital São Rafael, Salvador, Av. São Rafael,2152, Salvador, Bahia, Brazil
5Centro de Pesquisa Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, BA, Brazil
6Departamento de Física, FFCLRP, Av. Bandeirantes 3900,Ribeirão Preto, São Paulo, Brazil
*Corresponding author:
Angela Kinoshita, Pró-Reitoria de Pesquisa e Pós Graduação, Universidade do Sagrado Coração, Rua Irmã Arminda 10-50, Bauru, São Paulo, Brazil, Tel +55(14) 2107-7069, Fax: +55(14) 2107-7112, E-mail: angelamitie@gmail.com, angela.kinoshita@usc.br
Int J Oral Dent Health, IJODH-2-032, (Volume 2, Issue 3), Research Article; ISSN: 2469-5734
Received: July 14, 2016 | Accepted: August 22, 2016 | Published: August 24, 2016
Citation: Belmonte GC, Holgado LA, de Labio RW, Rosa PS, Segato R, et al. (2016) The Potential of Human Dental Pulp Stem Cell from Deciduous Teeth in Bone Regeneration: An Experimental Study in Rabbits. Int J Oral Dent Health 2:032. 10.23937/2469-5734/1510032
Copyright: © 2016 Belmonte GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To analyze the results of a bone regenerative procedure using stem cells derived from the dental pulp of human deciduous teeth (CDLH1) in combination with autologous platelet-rich plasma (PRP) in comparison to PRP alone, in the treatment of bone defects in rabbit calvaria.
Methods: In vitro tests were performed by flow cytometry and immunofluorescence for phenotypic characterization of CDLH1. Fourteen New Zealand rabbits were used in the in vivo experiment. Bilateral bicortical bone defects of 10 mm diameter were created in the skull. One of them was filled with CDLH1/PRP, while the other was filled with PRP only. Histomorphometry and CT images were used to evaluate bone regeneration at 7 and 60 days postoperatively, and results were statistically analyzed using paired Student's t-test.
Results: In vitro tests confirmed the presence of STRO-1 positive cells, which is a stem cell marker. In addition, CDLH1 were positive to CD44 and CD90 confirming their mesenchymal feature, and also expressed markers for differentiated cells: collagen type I, myosin, myelin and GFAP, and displayed tri-lineage differentiation potential, compatible with multipotency. Three-Dimensional CT images at 60 days postoperatively showed bone tissue completely covering the defect floor in the in defects treated with CDLH1/PRP, while defects treated with PRP still showed radiolucent areas. Histomorphometric analysis showed that, CDLH1/PRP produced a higher proportion (84 ± 8%) of formed bone compared to PRP (70 ± 10%) (p < 0.05).
Conclusions: The results demonstrated that stem cells derived from the pulp of deciduous teeth possess osteogenic potential, and that their use should be considered in the treatment of bone defects.
Keywords
Stem cells, Dental pulp, Platelet-rich plasma, Bone regeneration, Craniotomy, Tissue engineering
Introduction
In recent decades, the search for better techniques and materials for the treatment of bone defects in dentistry has been intense. As a result of several studies, many techniques have been described, and a wide variety of filling materials have been suggested to enhance and accelerate bone regeneration [1]. Such materials, known as graft materials, may be obtained from individuals themselves (autologous) or from an external source of natural or synthetic origin (heterogeneous), which can be used in the form of blocks or particles [2,3].
Bone defects may result from oncologic surgeries, acute or chronic infections, trauma or congenital malformations. Defects with greater magnitude, also called critical size defects, represent a major challenge in reconstructive and regenerative therapy [4-6]. The concept of critical size defect was established by Schmitz & Holinger [7] and refers to a bone defect which exceeds a certain size, preventing healing by regeneration and leading to the proliferation of fibrous tissue.
Materials used in reconstructive procedures, or bone regeneration, must meet a series of requirements in terms of safety and biocompatibility. Their morphology should facilitate revascularization, they should be easily incorporated into the recipient site, biodegradable and also present osteogenic potential [8,9]. Currently, the materials that best meet these requirements are of autologous origin. However, its acquisition requires a surgical procedure to a new site, which may result in increased surgical time, donor area morbidity and risk of infections [4]. It may also lead to local sequelae such as paresthesia, muscle and vascular lesions, edema formation, hematoma, and chronic pain [10]. Risks such as these encourage the search for new materials with properties similar to autologous bone, but of alternative origin. Platelet-rich plasma (PRP) is a therapeutic resource that has been used in reconstructive and regenerative bone surgery [11]. PRP is a product obtained from the laboratorial process of autologous blood, which is harvested in the preoperative period, rich in growth factors originated from platelet alpha granules [12]. It is an organic, non-toxic and non-immunoreactive product that has been used to accelerate wound healing [12,13]. The concentration of platelets with its growth factors is able to start undifferentiated bone cells activity earlier [14].
The ability of mesenchymal stem cells to differentiate into several tissue lineages, including bone tissue, has been demonstrated [15,16]. Stem cells are undifferentiated cells able to multiply, keeping their undifferentiated state, providing an active replacement to cell population in tissues. It is known that stem cells in various tissues have a regenerative role when these suffer from injury, trauma or diseases [17,18]. Studies have reported on human stem cells being harvested from the umbilical cord, amniotic fluid, bone marrow (BMMSCs) and adipose (ASCs), neurological and dental tissues (SHED) [19,20], as well as dental pulp (DPSC) [21-23] and periodontal ligament [24,25]. Some stem cell populations have been used in the regeneration of oral and craniofacial structures [21,25-30]. Recently, Kawashima (2012) [31] described the characteristics of dental pulp stem cells and the challenges for their use in tissue regeneration. As a result, some clinical studies have suggested their use in the treatment of large bone defects [32,33].
Therefore, the objective of this study was to investigate the regenerative (osteogenic) potential of human mesenchymal stem cells obtained from the dental pulp of deciduous teeth in combination with autologous PRP in bone defects artificially created by osteotomy in the calvaria of rabbits.
Material and Methods
This study was previously approved by the Ethics Committee of Universidade Sagrado Coração, Bauru, São Paulo, Brazil, and was conducted according to the recommendations set forth by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes [34].
Fourteen male New Zealand rabbits averaging 3 kg and six months of age were used in this study. During the experimental period, the animals were kept in standardized individual cages in good conditions of hygiene, food, and illumination.
CDLH1 mesenchymal stem cells
Stem cells from the pulp of human deciduous teeth (CDLH1) were isolated and cultured. The pulp tissue within teeth previously selected for exodontia was extracted with endodontic files inside a laminar flow, and placed in culture bottles with DMEM with 10% fetal bovine serum and 50 mg/ml gentamicin (complete DMEM). After the confluence of the adherent cell population, the culture was trypsinized and some of the cells were separated for cryopreservation. The phenotypic characterization of the mesenchymal stem cells from the pulp of human deciduous teeth (CDLH1) was performed by flow cytometry and immunofluorescence. Potency analysis was performed by tri-lineage differentiation assays.
Flow cytometry
Adherent cells in the sixth passage (P6) were selected for the detection of surface and intracellular antigens. The antibodies used are listed in table 1. Streptavidin PE-Cy5.5 diluted 1:100 (BD Biosciences) was used in the tubes stained with biotin-conjugated antibody. Data acquisition and analysis were performed in a FACS Calibur flow cytometer (Becton Dickinson, San Diego, CA) using the Cell Quest program. At least 50,000 events were collected and analyzed. Unlabeled antibodies cells were used as controls.
![]()
Table 1: List of antibodies used for flow cytometry.
View Table 1
Immunofluorescence
CDLH1 cells in the sixth passage were used for phenotypic characterization by immunofluorescence. The following antibodies were used: Anti-CD44 (Zymed), anti-CD45 (BioSource) Anti-CD90 (BD Bioscences), anti-CD105 (BD Bioscences) Anti-CD73 (BD Bioscences), anti-CD117 (Abcam), anti-CD133 (Abcam) Anti -aMyosin (Sigma Aldrich), Anti-Collagen type I (Santa Cruz Biotechnology), anti-GFAP (DAKO), Anti-Myelin (Zymed).
Tri-lineage differentiation assays
The differentiation of MSCs of the deciduous tooth pulp into adipocytes, osteocytes and chondrocytes was performed by using comercially availabe media supplemented with differentiation-inducing factors (StemPro adipogenesis differentiation kit and StemPro osteogenic differentiation kit, ThermoFisher Scientific), following the manufacturer's intructions. Adipogenic differentiation was visualized by Oil Red staining, while is the deposition of calcium as a result of osteogenic differentiation was observed by staining with Von Kossa method. For chondrogenic differentiation, the cells were grown in culture in DMEM and 10% FBS in 24-well plates. After reaching 40-50% confluency medium it was all replaced by differentiation medium consisting of DMEM supplemented with 100 μg/ml sodium pyruvate (Vetec), 40 ug/ml proline, 50 mg/ml L-ascorbic acid 2 phosphate (Sigma-Aldrich), 1 mg/ml BSA, 1 x insulin-transferrin-selenium plus (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich) and 10 ng/ml TGFβ3 (Sigma-Aldrich). After 21 days, cells were fixed with paraformaldehyde (PFA) 4% (Electron Microscopy Sciences) for 30 minutes, washed with PBS and stained with Alcian Blue solution (Gibco) in 0.1N HCl for 30 minutes. The cells were washed 3 times with 0.1N HCl solution, distilled water was added for the neutralization of the acidity. In all differentiation assays, a control group was maintained cultured in DMEM supplemented with 10% FBS.
Cell culture and processing for cytogenetic analysis
CDLH1 cells (passages 3 and 4) were grown in α-MEM (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% glutamine (Gibco), 1% antibiotic-antimycotic (Gibco) at 37°C and 5% CO2. Exchange of the maintaining medium during culture was performed at every three days. When cells reached confluence and cell division was active, block division of the mitotic metaphase was carried out through the use of 0.1 mg/ml colcemid (Gibco). Cells were then detached from the growth surface with 0.25% trypsin (Gibco) and treated with 0.057 M hypotonic solution (Merck). Cells were fixed using 3:1 methanol/acetic acid (Merck), to prepare slides of appropriate cell density with the dripping material. The temperature was controlled to 25 ± 2°C, and relative humidity to around 45-50%. Mounted, slides were then aged at 90°C for one hour [35].
The G-banding technique was performed under the same temperature and relative humidity conditions, according to the following procedure: Slides were treated with 0.06 M phosphate buffer (Merck) at 37°C and rinsed in running water. Staining was performed with Wright's stain (Merck) diluted in 0.06 M phosphate buffer (1:3). Finally, slides were rinsed again with tap water and dried at room temperature [36]. Under a magnification of 1,000X, approximately 20 metaphases of each sample were analyzed for the presence of numerical and/or structural cytogenetic abnormalities, according to the International System for Chromosome Nomenclature [37]. Images were captured via software and Image Pro Band View Plus.
After treatment with trypsin, samples were immediately transferred to a tube containing α-MEM (Gibco) medium supplemented with 10% human serum AB type to maintain viability, in preparation for surgeries.
Cell viability
Before using CDLH1 in the surgeries, cell viability was checked with Trypan blue reagent. The sample was considered suitable for use if at least 80% of cells were found to be viable.
Anesthesia
Animals underwent general anesthesia with the administration of 10.0 mg/kg body weight IM of xylazine hydrochloride (Anadesan, Vetbrands, Brazil), followed by the administration of 30.0 mg/kg body weight IM of ketamine hydrochloride (Dopalen, Vetbrands, Brazil).
After anesthetic onset, the skull and the chest portion of the animals were shaved, followed by topic antisepsis with 10% iodopovidone solution (Riodeine - Rioquímica Famacêutica Industry, Brazil).
PRP preparation
A volume of 9 mL of blood was obtained by cardiac puncture using a 10 mL syringe containing 1 mL of sodium citrate (3.2%) and used to PRP preparation, according to the protocol described by Parasuraman, et al. [38]. Initially, the blood was subjected to centrifugation (Eppendorf 5702 A) at 200 g for 10 minutes at a temperature of 23°C to promote the separation of blood cells. The supernatant plasma consisting of platelets and leukocytes were transferred to a new tube and subjected to a second centrifugation at 400 g for 10 minutes at a temperature of 23°C to concentrate the platelets, obtaining a concentration of at least 1.000.000/μL. Finally, the supernatant was withdrawn until the final volume of 800 μL of PRP was obtained.
Mesenchymal stem cells CDLH1 (P3 and P4) were added to PRP. Clot activation for PRP gel preparation was initiated with the addition of 10% calcium gluconate (Isofarma, Precabura Eusébio/CE, Brazil) immediately before their use in surgery.
Surgical procedures
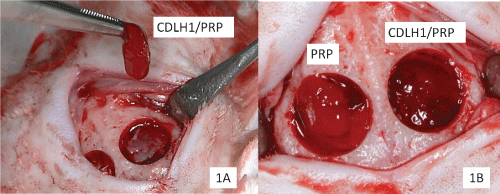
The area of skull to be operated was isolated by sterile surgical drapes. A skin incision in the sagittal plane from the occipital region to the frontonasal region of approximately 4.0 cm was performed and the tissue was separated. Then, the periosteum was incised exposing the calvaria. Bicortical bone defects were made on both sides, separated by the sagittal suture, under abundant irrigation with sterile saline using a trephine drill with 10 mm diameter (Neodent - Curitiba/PR, Brazil). The bone was removed, taking care not to injure the dura mater membrane (Figure 1A). The defect on the left side of the animal was then filled with CDLH1 suspended in PRP, while the other, on the right side, was filled with PRP (Figure 1B).
.
Figure 1: (A) Defects and treatment in a rabbit skull, showing the placement of CDLH1/PRP in the test defect; (B) Test and control defects treated with CDLH1/PRP (left) and PRP (right).
View Figure 1
The sutures were performed in layers; first the periosteum with a 4-0 absorbable suture (Vycril - Ethicon - São José dos Campos/SP), followed by the skin with a 4-0 nonabsorbable silk suture (Ethicon, São José dos Campos/SP). After surgery, antimicrobial prophylaxis was performed using enrofloxacin 10.0 mg/kg body weight IM (Flotril 2.5%-Schering-Plough) and 160 mg/kg of dipyrone (D-500-Fort Dodge) intramuscularly. The analgesic treatment continued for 3 consecutive days.
The animals were clinically assessed during the experimental period. A total of seven animals were euthanized at 7 days, and the other seven at 60 days postoperatively with an overdose (120 mg/kg) of general anesthetic. Samples containing the bone defects were excised and preserved in 10% buffered paraformaldehyde for tomographic and microscopic analyses.
Tomographic images
A Philips Brilliance Big Bore CT scanner was used to obtain CT images of specimens at 60 days. Axial images were obtained with 0.3 mm thick and 133 mm diameter reconstruction, 800 s exposure time, tube current of 75 mA, 60 mAs exposure, 110 KV voltage. Three-D images were reconstructed using a Hounsfield unities bone tissue window, i.e., allowing only bone tissue visualization.
Microscopic analysis
Samples were fixed in 10% buffered paraformaldehyde for 72 hours, decalcified with 18% ethilenodiaminotetraacetic (EDTA) acid solution. After decalcification, samples were firstly cut to separate the defects and then, perpendicularly to the midsagittal suture in the central part of defects. After embedded in paraffin, three sections of the center of the surgical defect with 6 m thick were obtained for microscopic analysis. Two of these sections were stained with Masson trichrome for histomorphometric assessment, while the remaining section was stained with H&E (Hematoxylin and Eosin) for the qualitative histologic assessment. A Nikkon H550L optical microscope coupled to a computer was used to acquire the images from the sections. Histomorphometry was performed by an examiner who was blinded in respect to the treatment. Bone and connective tissue areas within the defects were measured using a 50 μm grid placed over the photomicrography, taking into account the original extension of the defect (10 mm). The results were expressed by the proportion (%) of the area occupied in relation to the total area of the defect. Paired Student's t-test was used to compare the results obtained from test defects (CDHL1/PRP) to those from control defects (PRP) at 7 and 60 days postoperatively.
Results
No animal developed any signs of clinically apparent infections in the defect areas or around them at any moment during the experimental period.
Cytogenetic analysis
Cytogenetic analysis of cultured CDLH1 P3 and P4 cells showed normal karyotype (46, XY), as shown in figure 2.
Characterization of stem cell line
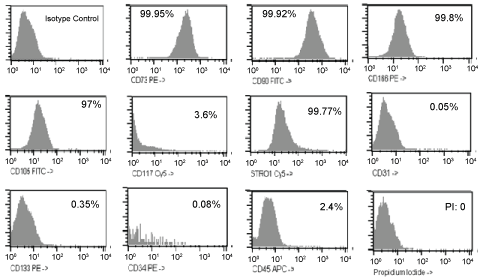
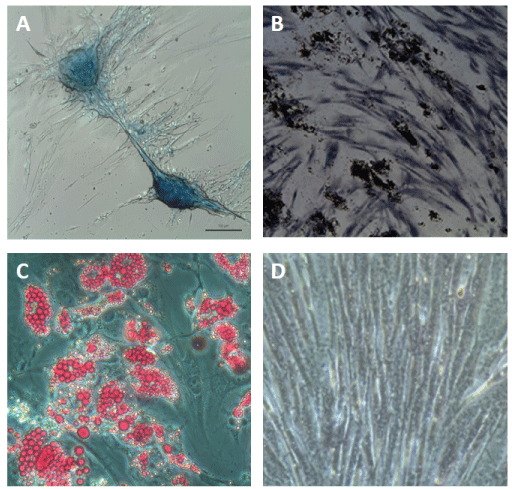
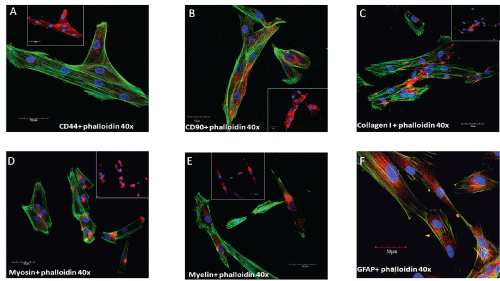
Flow cytometry showed cells with high expression of surface markers characteristic of mesenchymal stem cells such as CD105, CD73, CD44, CD90, CD166, and STRO-1 (Figure 3). The differentiation of MSCs of the deciduous tooth pulp into adipocytes, osteocytes and chondrocytes are showed in figure 4. Moreover, the markers for differentiate cell type α-myosin, myelin, GFAP, and type I collagen, were also found to be present by immunofluorescence analysis (Figure 5). The expression was low or absent for the hematopoietic lineage markers CD117, CD133, CD31, CD38 and CD45.
.
Figure 3: Flow cytometry analysis. Frequency of expression of surface markers in CDLH1 culture in the 6th passage.
View Figure 3
.
Figure 4: Characterization of multipotency of CDLH1 by differentiation assays. (A) Chrondrogenic differentiation is confirmed by Alcian blue staining for proteoglycans synthesis; (B) Osteogenic differentiation confirmed by visualization of calcium deposits with Von Kossa staining; (C) Adipogenic differentiation confirmed by visualization of intracellular lipids staining with Oil Red; (D) Phase-contrast image of a control undifferentiated stem cell culture.
View Figure 4
CT images
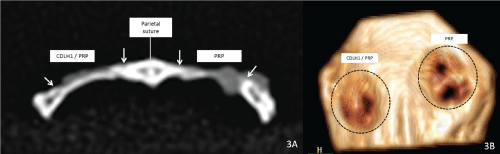
Figure 6A and figure 6B show, respectively, tomographic images of a section near the central region of the defect, and the 3D reconstruction of a sample taken at 60 days. The presence of bone tissue in the defect region could be noted, characterized by areas of higher radiopacity. In defects treated with CDLH1/PRP (left), bone tissue was completely covering the defect floor connecting the edges. Defects treated with PRP, on the other hand, presented radiolucent areas.
.
Figure 5: Immunophenotype of CDLH1 stem cells showing the markers CD44 and CD90 (A,B); characterizing mesenchymal stem cells, and the markers for collagen I, myosin, myelin and GFAP (C,D, E,F); characterizing multipotency.
View Figure 5
.
Figure 6: (A) CT image of a specimen at 60 days postoperatively; test defect (left), control defect (right). Greater radiopacity can be seen in the test defect, indicating greater proportion of bone tissue within the defect area; (B) 3D-CT image of a 60-day specimen; the test defect (left) presents a larger amount of bone inside the defect compared to control (right).
View Figure 6
Microscopic analysis
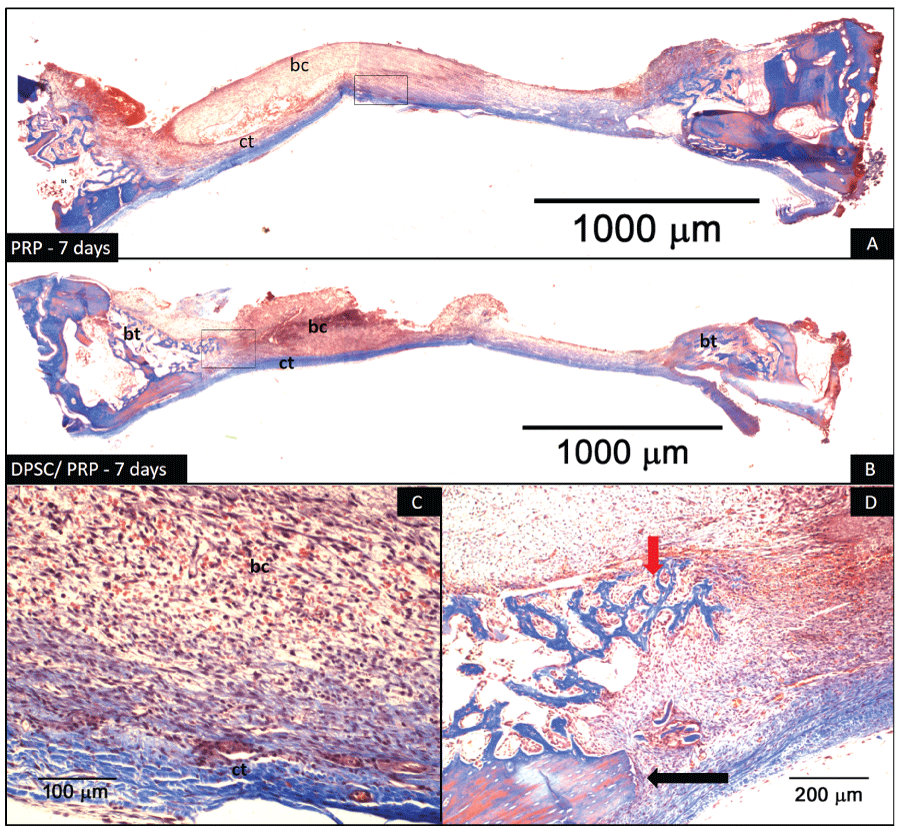
7 days postoperatively: Bone defects presented a healing pattern typical of large defects. In this phase, control specimens treated with PRP exhibited a large amount of blood clot, still under differentiation, especially in the upper portion of the defect, and the presence of small foci of connective tissue near the edges and in the regions above the dura mater (Figure 7A and Figure 7C). Test specimens treated with CDLH1/PRP showed characteristics similar to those described in the control specimens, but in a more advanced stage, in which it was possible to observe increased formation of immature trabecular bone at the margins of defects (Figure 7B and Figure 7D).
.
Figure 7: Photomicrography of defects 7 days postoperatively. (A) Defect treated with PRP; (B) Defect treated with CDLH1/PRP. The arrows delineate the extent of the defect. In (A) the blood clot (bc) can be observed along the entire length at the top of the defect. Over the dura mater there is a narrow band of connective tissue (ct); (B) Blood clot (bc) can be seen in the upper portion of the defect, and connective tissue (ct) covering the entire length of the lower portion. There is trabecular bone tissue (bt) formation at the margins of the defect (Masson Trichrome. 2X original linear magnification); (C) Same image from (A) at higher magnification, revealing the band of differentiating blood clot (bc) (red color) and connective tissue (ct) (blue color) (Masson's trichrome. 20X original linear magnification); (D) Same image from (B) at higher magnification, revealing the presence of bone trabeculae growing from the margins of the defect towards the center (red arrow). At the bottom right, there is some remaining lamellar bone tissue (black arrow) (Masson's trichrome. 10X original linear magnification).
View Figure 7
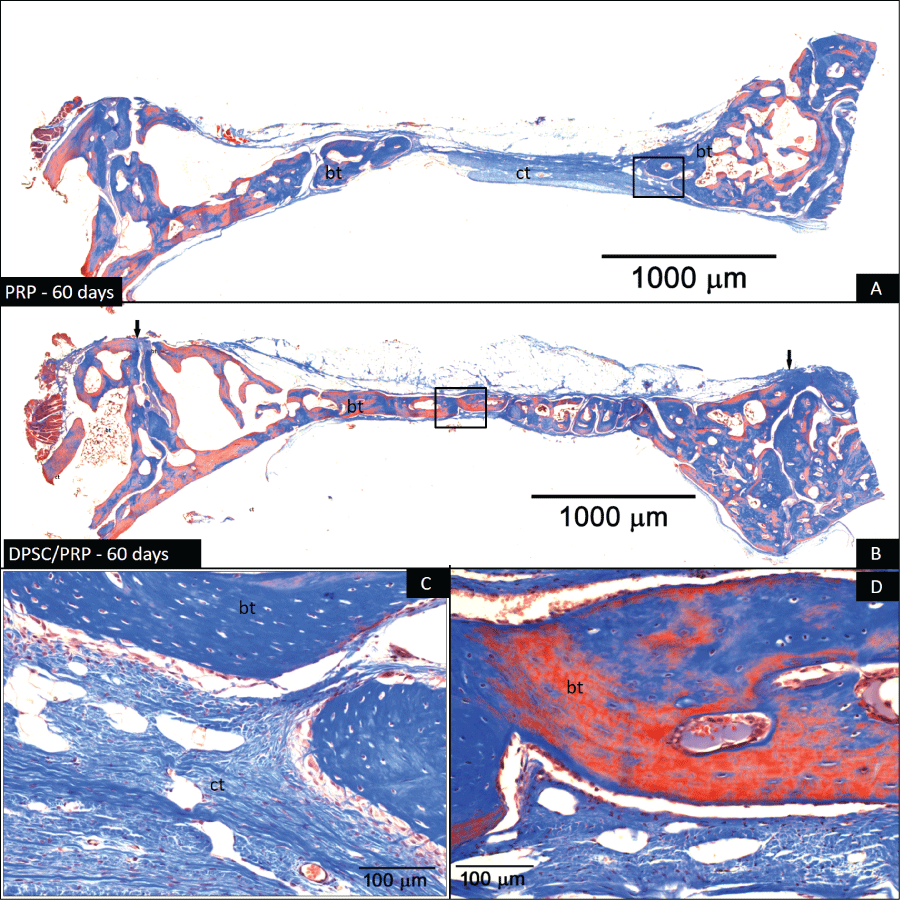
60 days postoperatively: At this stage, bone formation in the defect areas could be observed more clearly. Control specimens exhibited the formation of nonlamellar bone callus in the remodeling stage (Figure 8A and Figure 8C). Connective tissue is present in the center of the defects. However, test specimens treated with CDLH1/PRP exhibited defects filled with bone tissue in an advanced stage of remodeling, with some regions exhibiting lamellar bone tissue (Figure 8B and Figure 8D).
.
Figure 8: Photomicrography of defects 60 days postoperatively. (A) Defect treated with PRP; (B) Defect treated with CDLH1/PRP. The arrows delineate the extent of the defect. In (A) the absence of blood clot within the defect and presence of bone tissue (bt) at the margins of the defect can be seen. In the center it is possible to observe connective tissue (ct) still in the differentiation process. In (B) the defect is completely filled with bone tissue (bt) (Masson Trichrome. 2X original linear magnification); (C) Same image from (A) at higher magnification revealing the presence of bone tissue (bt) at the edges of the defect. Note the presence of connective tissue (ct) permeated with cells around the bone callus; (D) Same image from (B) at higher magnification in the margin of the defect. Note the presence of lamellar bone (bt). There are osteocytes in large numbers (Masson's trichrome. 20X original linear magnification).
View Figure 8
Histomorphometry: Figure 9A and figure 9B show the results of bone and connective tissue quantitative measurements at 7 and 60 days postoperatively. Statistically significant differences between treatments were found at 60 days postoperatively. Defects filled with CDLH1/PRP produced a higher percentage (84 ± 8%) of formed bone in comparison with those filled with PRP (70 ± 10%) (p < 0.05).
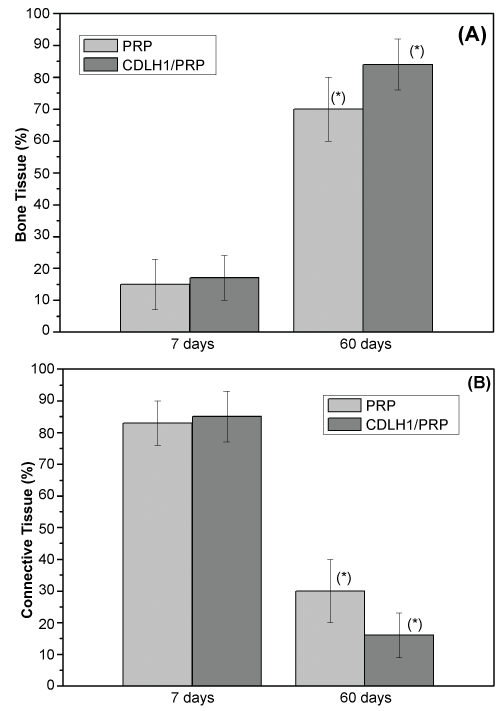
.
Figure 9: Histomorphometric assessment of (A) bone; (B) connective tissue, at 7 and 60 days postoperatively. A statistically significant difference between treatments was present at 60 days (*p < 0.05. Paired Student's t-test).
View Figure 9
Discussion
This study evaluated the regenerative potential of human stem cells obtained from the pulp of deciduous teeth in bone defects artificially created in the skull of rabbits. The in vitro tests confirmed the mesenchymal characteristics of CDLH1. Therefore, heterogeneous cells in combination with autologous PRP were used to treat bone defects. Autologous materials associated with many different types of heterogeneous materials, allografts, or alloplastic have been described in the literature for decades, showing satisfactory results in the treatment of bone defects [3,10,39,40].
The ossification of the calvaria, as well as the maxilla and mandible, occurs by intra-membranous ossification. A major obstacle in bone regeneration is the proliferation of connective tissue into the wound healing defect. It is assumed that fibroblasts release soluble factors inhibiting osteogenesis. Cell failure in the formation of a calcificable bone matrix probably occurs due to the release of inadequate quantities of growth and differentiation [41].
In this study as well as Yamada, et al. [21], autologous PRP was used as a vehicle for the immersion of CDLH1 stem cells (Figure 1A). In addition, the periosteum was sutured over the defects in order to act as a barrier against the proliferation of fibrous tissue into the defects.
PRP is a source of growth factors important in the healing of both soft and hard tissues [42]. Among the growth factors released by platelets in PRP, platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-b), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin growth factor-I (IGF-I) and fibroblast growth factor (bFGF) are the most important [43,44]. In the last decade, extensive use of PRP alone, or in combination with other osteopromotor materials in bone grafting procedures used in the dental prosthetic rehabilitation with osseointegrated implants have been reported [13,42,45].
Some studies have shown controversial results regarding the induction potential of PRP [46-50]. Such controversy seems to be associated with variations in protocols for the production and preparation of PRP [51].
In this study the proportion of newly formed bone in defects treated with PRP alone 60 days postoperatively was found to be 70%. However, all specimens also presented connective tissue in large quantities within the defect (Figure 8A and Figure 8C), i.e., none of these defects was fully regenerated. Similar results were found by [44,52] in the treatment of bone defects created in calvaria of rats with PRP. Other studies using the same animal model and period found smaller proportions of bone formation than those found in the defects treated with PRP and CDLH1/PRP in this study. Tamimi, et al. [53] found that the defects treated with calcium phosphate presented 21% of the total area filled with the biomaterial and 53% with formed bone 60 days postoperatively.
Mesenchymal stem cells exhibit the potential to differentiate in a variety of cells such as osteoblasts, myoblasts, chondrocytes and adipocytes. Some studies have reported that the proliferation potential of stem cells obtained from the pulp of deciduous teeth is similar to that of stem cells isolated from bone marrow [20,54,55], suggesting their use in regenerative treatment of bone defects [24]. Zheng, et al. [56] evaluated the regenerative potential of stem cells isolated from the pulp of deciduous incisors of pigs in the treatment of bone defects with critical dimension in the jaw of those same animals. Their histological and immunohistochemical results as well as the tomographic images showed that the group of animals treated with stem cells had a higher proportion of newly formed bone in the defect area compared to animals treated with B-Tricalcium Phosphate, and to animals that received blood clot as a filling material. The ability of dental pulp stem cells to form bone was also demonstrated by Yamada, et al. [21] in canine animal model.
In this study, the results obtained from the defects treated with the immersion of stem cells in PRP showed in the later period (60 days) a smaller proportion of remaining connective tissue and a larger proportion of bone tissue than those defects treated with PRP only (Figure 8 and Figure 9). The tomographic analyses (Figure 6A and Figure 6B) confirmed the microscopic results, revealing bone tissue in greater proportion in specimens treated with CDLH1. The histomorphometric assessment (Figure 9) shows that both treatments stimulated bone formation in a very similar way in the early stages (7 days), but bone formation was significantly higher in the specimens treated with CDLH1 at 60 days postoperatively.
Conclusion
The results obtained in this study demonstrated that human stem cells derived from the pulp of deciduous teeth CDLH1 present osteogenic potential. This may constitute an effective and minimally traumatic option for the treatment of bone defects.
Competing Interests
There are no conflicts of interest.
Acknowledgements
The authors would also like to thank Mr. Wilson Orcini, Ms. Maira Couto and Ms Elizalva T. Guimarães for their technical support, and Mr Antonio Carlos Correa for reviewing the English version of the paper and Hospital das Clinicas de Ribeirão Preto, Universidade de São Paulo, for use of CT scanner.
References
-
Kawamura M, Urist MR (1988) Induction of callus formation by implants of bone morphogenetic protein and associated bone matrix noncollagenous proteins. Clin Orthop Relat Res 236: 240-248.
-
Ziran BH, Smith WR, Morgan SJ (2007) Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. J Trauma 63: 1324-1328.
-
Buser D (2009) 20 Years of Guided Bone Regeneration in Implant Dentistry. 2nd ed. Quitenssence Publishing Co, Chicago.
-
Friedlaender GE (1987) Bone grafts. The basic science rationale for clinical applications. J Bone Joint Surg Am 69: 786-790.
-
Goldberg VM, Stevenson S (1987) Natural history of autografts and allografts. Clin Orthop Relat Res: 7-16.
-
Lane JM, Sandhu HS (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18: 213-225.
-
Schmitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res: 299-308.
-
Schlegel KA, Sindet-Pedersen S, Hoepffner HJ (2000) Clinical and histological findings in guided bone regeneration (GBR) around titanium dental implants with autogeneous bone chips using a new resorbable membrane. J Biomed Mater Res 53: 392-399.
-
Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, et al. (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27: 3230-3237.
-
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA (1996) Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res: 300-309.
-
Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55: 1294-1299.
-
Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, et al. (1989) A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol 16: 545-548.
-
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14: 529-535.
-
Marx RE, Garg AK (1999) Bone graft physiology with use of platelet-rich plasma and hyperbaric oxygen: The Sinus Bone Graft, Colorado Quintessence Books, 183-189.
-
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, et al. (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98: 2396-2402.
-
Lemischka IR (2005) Stem cell biology: a view toward the future. Ann N Y Acad Sci 1044: 132-138.
-
Blau HM, Brazelton TR, Weimann JM (2001) The evolving concept of a stem cell: entity or function? Cell 105: 829-841.
-
Fodor WL (2003) Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate. Reprod Biol Endocrinol 1: 102.
-
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
-
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97: 13625-13630
-
Yamada Y, Nakamura S, Ito K, Sugito T, Yoshimi R, et al. (2010) A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng Part A 16: 1891-1900
-
Guimarães ET, Cruz GS, de Jesus AA, Lacerda de Carvalho AF, Rogatto SR, et al. (2011) Mesenchymal and embryonic characteristics of stem cells obtained from mouse dental pulp. Arch Oral Biol 56: 1247-1255.
-
Wang X, Sha XJ, Li GH, Yang FS, Ji K, et al. (2012) Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol 57: 1231-1240.
-
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149-155.
-
Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, et al. (2006) Craniofacial tissue engineering by stem cells. J Dent Res 85: 966-979.
-
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9: 641-650.
-
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100: 5807-5812.
-
Alhadlaq A, Mao JJ (2004) Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 13: 436-448.
-
Marion NW, Mao JJ (2006) Mesenchymal stem cells and tissue engineering. Methods Enzymol 420: 339-361.
-
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100: 1249-1260.
-
Kawashima N (2012) Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol 57: 1439-1458.
-
Ueda M, Yamada Y, Ozawa R, Okazaki Y (2005) Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. Int J Periodontics Restorative Dent 25: 129-137.
-
Hibi H, Yamada Y, Ueda M, Endo Y (2006) Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg 35: 551-555.
-
European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes.
-
Barch MJ, Knutesen T, Spurbech JL (1997) The AGT Cytogenetics Laboratory Manual (3rd edn), Lippincott-Raven Philadelphia, USA.
-
Sanchez O, Escobar JI, Yunis JJ (1973) A simple G-banding technique. Lancet 302: 269.
-
Shaffer LG, Slovac ML, Campbell LJ (2009) ISCN: An International System for Human Cytogenetic Nomenclature. Basel: Karger.
-
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1: 87–93.
-
Kamoda H, Yamashita M, Ishikawa T, Miyagi M, Arai G, et al. (2012) Platelet-Rich Plasma Combined With Hydroxyapatite for Lumbar Interbody Fusion Promoted Bone Formation and Decreased an Inflammatory Pain Neuropeptide in Rats, Spine 37: 1727-1733
-
Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84: 454-464.
-
Schmitz JP, Schwartz Z, Hollinger JO, Boyan BD (1990) Characterization of rat calvarial nonunion defects. Acta Anat (Basel) 138: 185-192.
-
Arora NS, Ramanayake T, Ren YF, Romanos GE (2009) Platelet-rich plasma: a literature review. Implant Dent 18: 303-310.
-
Schilephake H (2002) Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg 31: 469-484.
-
Plachokova AS, van den Dolder J, van den Beucken JJJP, Jansen JA (2009) Bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Surg 38: 861-869.
-
Aghaloo TL, Moy PK, Freymiller EG (2004) Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants 19: 59-65.
-
Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, et al. (2002) Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res 37: 300-306.
-
Fennis JP, Stoelinga PJ, Jansen JA (2004) Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg 33: 48-55.
-
Roldán JC, Jepsen S, Miller J, Freitag S, Rueger DC, et al. (2004) Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 34: 80-90.
-
Thorwarth M, Wehrhan F, Schultze-Mosgau S, Wiltfang J, Schlegel KA (2006) PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone 38: 30-40.
-
Nagata MJ, Melo LG, Messora MR, Bomfim SR, Fucini SE, et al. (2009) Effect of platelet-rich plasma on bone healing of autogenous bone grafts in critical-size defects. J Clin Periodontol 36: 775-783.
-
Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH (2008) Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res 19: 539-545.
-
Plachokova AS, van den Dolder J, Stoelinga PJ, Jansen JA (2007) Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res 18: 244-251.
-
Tamimi F, Torres J, Kathan C, Baca R, Clemente C, et al. (2008) Bone regeneration in rabbit calvaria with novel monetite granules. J Biomed Mater Res A 87: 980-985.
-
Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, et al. (2006) Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38: 758-768.
-
Sloan AJ, Waddington RJ (2009) Dental pulp stem cells: what, where, how? Int J Paediatr Dent 19: 61-70.
-
Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, et al. (2009) Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res 88: 249-254.