Journal of Dermatology Research and Therapy
Dermatitis Herpetiformis: A Cutaneous Gluten-Related Disorder with Possible Exception in Asian Patients
Chika Ohata*
Department of Dermatology, Kurume University School of Medicine, Japan
*Corresponding author:
Chika Ohata, MD, PhD, Department of Dermatology, Kurume University School of Medicine, 67 Asahimachi, Kurume, Fukuoka 830-0011, Japan, Tel: +81-942-31-7571, Fax: +81-942-34-2620, E-mail: ohata@med.kurume-u.ac.jp
J Dermatol Res Ther, JDRT-2-039, (Volume 2, Issue 6), Mini Review; ISSN: 2469-5750
Received: October 27, 2016 | Accepted: November 28, 2016 | Published: November 30, 2016
Citation: Ohata C (2016) Dermatitis Herpetiformis: A Cutaneous Gluten-Related Disorder with Possible Exception in Asian Patients. J Dermatol Res Ther 2:039. 10.23937/2469-5750/1510039
Copyright: © 2016 Ohata C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Dermatitis herpetiformis (DH) is an autoimmune bullous disease characterized by intensely pruritic, chronic, and recurrent vesicles on extensor surfaces such as the elbows, knees, and buttocks. The collection of neutrophils at the papillary tips is the typical histopathological finding, and a characteristic diagnostic feature is granular immunoglobulin A deposition in the papillary dermis by direct immunofluorescence. DH is closely associated with gluten sensitive enteropathy and is considered a cutaneous manifestation of gluten sensitivity; i.e., an extra-intestinal presentation of celiac disease. Gluten free diet is the first-line therapy for patients with DH and dapsone is also effective. DH preferentially affects Caucasians who carry human leukocyte antigen (HLA)-DQ2 or HLA-DQ8. The major autoantigen is epidermal transglutaminase. This review focuses on the confirmedfeatures of DH and our recent findings specific to DH in Japanese patients.
Keywords
Dermatitis herpetiformis, Gluten sensitive enteropathy, Celiac disease, Gluten free diet, Epidermal transglutaminase
Introduction
Dermatitis herpetiformis (DH) was first reported by Duhring in 1884 [1]. Patients with DH develop intensely pruritic papulovesicular skin lesions predominantly on the elbows, knees, and buttocks [2]. DH is associated with enteropathy, and both are caused by gluten intake. Celiac disease (CD), another gluten sensitivity disease, and DH have common characteristics such as close association with human leukocyte antigen (HLA)-DQ2 and HLA-DQ8, and immunoglobulin (Ig) A autoantibodies to tissue transglutaminase (tTG: transglutaminase 2) and epidermal transglutaminase (eTG: transglutaminase 3) [3,4]. Therefore, DH is considered an extra-intestinal presentation of CD. This review details recent advances in understanding the pathogenesis, clinical manifestations, diagnosis, and treatment of DH.
Epidemiology
DH is most prevalent among the Caucasian population, particularly those of northern European descent, including the North American population. The prevalence ranges from 10 to 39 per 1,000,000 individuals [5-7]. By contrast, DH is rare among Asian and African populations [8,9]. DH commonly develops in the second, third, and fourth decades, but it may appearin children or aging adults [7,8,10-12]. DH is more common in men than women, with a male-to-female ratio of 1.5:1 [7,8]. DH closely associated with gluten sensitive enteropathy (GSE), the symptoms of which range from severe to silent [13]. However, small bowel biopsy is unnecessary for diagnosing DH because the disease is the cutaneous counterpart of CD [2,14]. Compared with the general population, DH patients have a higher incidence of CD and DH among first-degree relatives. A Finnish population study reported that 13.5% and 4.6% of DH patients have first-degree relatives affected with CD and DH, respectively [15]. In contrast to Caucasian patients, Japanese DH patients usually do not have GSE [8,9]. However, controversy exists regarding the rarity of GSE in Japanese DH patients because a few patients were reported to have villous atrophy in a small bowel biopsyand small intestine of most of Japanese DH patients were not examined endoscopically nor histopathologically [8]. Moreover, GSE can be asymptomatic in Caucasian DH patients [11]. More knowledge about the small bowel mucosal alterations may be required to fully elucidate the association between Japanese DH patients and GSE, but most of Japanese DH patients, particularly who do not have abdominal symptoms, usually refuse examinations of small intestine.
Pathogenesis
Patients with both DH and CD have circulating IgA autoantibodies against transglutaminase. Intestinal damage caused by gluten exposure is suggested to produce IgA anti-tTG and anti-eTG antibodies [16]. tTG and eTG are highly homologous within enzymatically active domains, which results in high cross-reactivity [14,17]. In a previous study of DH, direct immunofluorescence (DIF) demonstrated colocalization of IgA and eTG, but not IgA and tTG, in the papillary dermis [18]. This result seems to be analogous to the colocalization of tTG and IgA in the small bowel in patients with CD [19]. Thus, tTG is the major autoantigen in CD [20], whereas eTG is the major autoantigen in DH [18,21,22]. Zone, et al. developed an animal model that reproduces the same immunopathological pattern of granular IgG deposition in the dermal papillae as that seen in DH by passive transfer of goat IgG anti-eTG antibodies to SCID mice engrafted with normal human skin [23]. Moreover, they reproduced the characteristic pattern of human granular IgA deposition in dermal papillae by transferring DH serum with high levels of IgA anti-eTG antibodies [23]. Because the only source of human eTG in this system is the human epidermis adjacent to the immune deposition. Zone, et al. concluded that eTG was released from keratinocytes into the papillary dermis, and this deposition process explained the negative indirect immunofluorescence results observed with DH serum.
A recent study showed that the number of regulatory T cells (Tregs) in DH skin lesions is significantly lowerthan that in healthy skin [24]. This reduction of Tregs may suppress the downregulation of the inflammation caused by the deposition of IgA anti-eTG antibodies in the papillary dermis. DH skin lesions show infiltration by CD4+ T helper (Th) cells that belong to the Th2 phenotype. Interleukin 4, interleukin 5, and granulocyte macrophage colony-stimulating factor secreted by Th2 cells are powerful chemotactic agents for neutrophils and eosinophils [25]. Neutrophilic infiltrate in the papillary dermis is suggested to lead to the release of cytokines, chemokines, and proteases as well as induce collagenases or stromelysin-1 in basal keratinocytes, which lead to blister formation [26,27].
Approximately 85% of patients with DH carry HLA-DQ2 (DQB1*02:01), and the majority of the remaining patients carry HLA-DQ8 (DQB1*03:02) [3]. The processing of the dietary gluten antigen (gliadin) requires HLA DQ2 or HLA DQ8 [28]. CD and DH in the Caucasian population virtually always occur with these HLA types. However, our study of DH in Japanese patients showed that only 37.5% carried HLA-DQ8 and none carried HLA-DQ2 [9]. The absence of HLA-DQ2 in Japanese DH patients is likely attributable to the virtual absence of HLA-DQ2 in the Japanese population [29]. CD is rare in Asian countries because both wheat consumption and the frequency of HLA-DQ2 are low [29]. These factors may also explain the low prevalence of DH in Asia.
Given that the prerequisite HLA type for gluten antigen processing is scarce in the Japanese population, gluten may be unlikely to initiate DH in Japanese patients [9]. Because no consistent evidence of underlying CDhas been found in Japanese patients with DH [8,9], factors other than gluten may be responsible for the IgA/eTG complexes observed in these patients. In addition, findings of patients who lack evidence of IgA/Etg complexes in either skin or serum suggest that other autoantigens play a role in DH in Japanese patients [9].
Clinical Manifestations
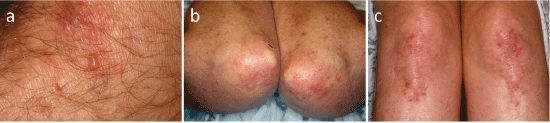
Severe pruritus is the principal clinical manifestation in DH. The characteristic clinical manifestations of the illness are grouped polymorphic lesions consisting of erythematous papules surmounted by vesicles, erosions, and excoriations (Figure 1a) [2,8]. Large bullae are unusual, and only crusted lesions may be observed without apparent vesicles. The most commonly involved sites are the extensor surfaces of the elbows (Figure 1b) and the knees (Figure 1c), as well as the shoulders, buttocks, sacral region, and face [2,8]. Most patients also have lesions in the scalp and the nucha. Lesions are symmetrical and heal with hyperpigmentation and/or hypopigmentation without scarring. Mucosal involvement may occur [30].
.
Figure 1: a) Erythema with vesicles and crust in dermatitis herpetiformis; b) Only erythema and hypopigmented lesionsare seen on the elbows; c) Grouped erythema, vesicles, and crusts on the knees. (Courtesy of Prof. Jason Bok Lee).
View Figure 1
Laboratory Tests
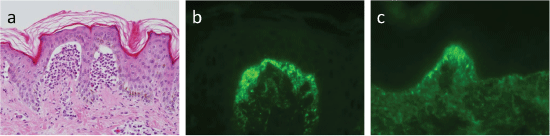
Biopsy of an early lesion shows collections of neutrophils at the papillary tips with occasional eosinophilic infiltrate (Figure 2a). DIF reveals non-linear (mostly granular, or fibrillar) IgA deposition in the papillary dermis (Figure 2b and Figure 2c) [31,32]. IgA deposition along the basement membrane zone maysometimes occur [2]. Compared with that in lesional skin, IgA deposition is greater in normal appearing perilesional skin, and these deposits disappearslowly with adherence to a strict gluten free diet (GFD) [32]. Complete resolution of IgA deposition may take several years [2]. Although the deposition in most Caucasian patients has a granular pattern, that in more than one-third of Japanese patients has afibrillar pattern [8]. IgG, IgM, and C3 are also deposited in the lower percentages [33]. Indirect immunofluorescence reveals no reactivity [23].
.
Figure 2: a) Neutrophils with a few eosinophils infiltrate in the papillary dermis, and give rise to subepidermal bullae (hematoxylin and eosin, × 200); b and c) Granular; (b) and fibrillar; (c) patterns of immunoglobulin a deposition in direct immunofluorescence.
View Figure 2
Tests for IgA antibodies to eTG, tTG, and endomysium are useful diagnostic tools for DH [2,16]. tTG is the major antigen for anti-endomysium antibodies [18]. eTG, rather than tTG, is considered the dominant autoantigen in DH [18]. IgA anti-eTG antibodies are of two types in DH. One type binds exclusively to eTG, and the other binds cross-reactively to both eTG and tTG [14]. High sensitivity (60-80%) and specificity (90-100%) of anti-eTG antibodies have been reported in Caucasian DH patients [34,35]. By contrast, the detection rate for IgA anti-tTG and anti-eTG antibodies is much lower in Japanese DH patients [9]. The levels of IgA anti-eTG and anti-tTG antibodies correlate with the degree of enteropathy [16]. One case study reported that IgA anti-eTG antibody levels decreased over time with adherence to GFD, which suggests that testing for IgA anti-eTG antibodies may be useful to monitor disease activity [35].
Comorbidities
DH is closely associated with gluten sensitivity [36]. Compared with CD patients, DH patients usually have milder gastrointestinal symptoms [37,38]. Atrophic gastritis sometimes occurs and may be a precursor to pernicious anemia [39]. Untreated patients may develop malabsorption, and consequently, anemia and bone loss [40]. Small bowel lymphoma can develop owing to GSE [41]. In addition, non-Hodgkin lymphoma can occur in patients with DH [42,43]. DH is associated with a number of autoimmune conditions, including thyroid disease [44], type I diabetes mellitus [45,46], and autoimmune connective tissue diseases such as Sjogren syndrome [45], rheumatoid arthritis [47] and lupus erythematosus [48]. Addison disease [45,49] and vitiligo [50,51] have also been associated with DH. Although neurologic diseases, including epilepsy, ataxia, and dementia, have been reported in patients with CD [52], no evidence to date supports an association between neurologic disease and DH [53]. Most Japanese DH patients have rarelyGSE, lymphoma or autoimmune diseases [8,9].
Differential Diagnosis
Because DH is characterized by polymorphic lesions, the differential diagnosis includes various skin diseases such as chronic eczema, atopic dermatitis, scabies, pemphigoid, and linear IgA bullous dermatosis. DIF differentiates DH from these diseases. A skin biopsy for DIF should be considered for refractory pruritic rashes even if no apparent gastrointestinal symptoms are found.
Recently described nonceliac gluten sensitivity (NCGS) may involve the skin [54]. The skin lesions of NCGS are pruritic and similar to those of subacute eczema, psoriasis, or DH [55]. A recent study identified C3 deposition at the basement membrane zone in a granular or micro-granular pattern in DIF of NCGS lesions despite the absences of specific histopathological changes [55]. Because the entity of NCGS remains controversial, further study is necessary to elucidate its characteristic skin mainfestations.
Treatment
GFD is the first-line therapy for DH [56-58], and may protect against the development of lymphoma. Because strict GFD adherence is time-consuming and requires extensive knowledge of food ingredients, patients should be encouraged to consult with a dietitian and join DH support groups. Gluten comprises the proline- and glutamine-rich proteins of wheat, barley, rye, and oat. However, a recent study revealed that oats can be safely consumed by individuals with DH [59-61], likely because (1) compared with the gluten-like molecules in the other cereals, those in oats have only two antigenic sequences, and (2) the amount of gluten in oats is much lower than that in the other cereals [62].
Because improvements in DH symptoms related to GFD adherence require a long period-months to years-to occur, patients are usually prescribed dapsone (25-150 mg/day) for fast control of pruritus and blister formation [63]. Although dapsone is effective in skin lesions because it suppressesthe migration of neutrophils to extravascular sites [64], it does not improve GSE. The adverse effects of dapsone administration, including hemolytic anemia, should be monitored. Other sulfonamide drugs have effects similar to those of dapsone and can be used in dapsone-intolerant patients [65,66].
Systemic corticosteroids are ineffective, whereas potent topical steroids are useful in decreasing pruritus. Although adherence to a GFD is the cornerstone of DH management in Caucasian patients, most Japanese DH patients improve without GFD [8,9]. This outcome may be attributable to the rare occurrence of GSE in Japanese DH patients, who can be successfully treated with dapsone with or without topical corticosteroids. Five patients required only several-month administration of dapsone to clear the skin lesions, and the lesions did not recur after ceasing dapsone [8]. If these patients had GSE, their skin lesions should have recurred after cessation of dapsone. These patients may well represent rare occurrence of GSE amongJapanese DH patients.
Prognosis
DH is a chronic disease that requires long-term GFD adherence. Those who can comply with good response are likely to have reduced mortality [67] and may be able to stop dapsone treatment. Although patients with CDwho respond poorly to a GFD may have high mortality and shortened survival time [68], DH patients who is non-responsive to a GFD have relatively favorable prognosis [69]. DH remission is recognized in approximately 10% of patients, most of whom developed DH at the age of 39 years or older [70].
Conclusions
Clinicians can use guidelines to optimize the diagnosis and management of DH. However, although several guidelines for DH have been published [2,71-73], only one is available in English [2]. These guidelines were established for Caucasian patients, in which DH is most commonly diagnosed. Because the features of DH in Japanese patients and Caucasian patients differ, particularly in the virtual absence of GSE, GFD is rarely necessaryto treat Japanese patients with DH. Additional studies of patients with DH in other Asian countries or in African countries would be useful to elucidate the features of DH further.
Acknowledgments
The author thanks Professor Jason Bok Lee (Thomas Jefferson University, Philadelphia, PA, USA) for providing clinical photographs. This work was supported by Health Labour Sciences Research Grant Number H26-077 (Research on Measures for Intractable Diseases).
Conflicts of Interest
The author declares no conflicts of interest.
References
-
Duhring LA (1983) Landmark article, Aug 30, 1884: Dermatitis herpetiformis. By Louis A. Duhring. JAMA 250: 212-216.
-
Caproni M, Antiga E, Melani L, Fabbri P, Italian Group for Cutaneous Immunopathology (2009) Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol 23: 633-638.
-
Spurkland A, Ingvarsson G, Falk ES, Knutsen I, Sollid LM, et al. (1997) Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA-DQ (alpha 1*0501, beta 1*02) or the HLA-DQ (alpha 1*03, beta 1*0302) heterodimers. Tissue Antigens 49: 29-34.
-
Sollid LM (2000) Molecular basis of celiac disease. Annu Rev Immunol 18: 53-81.
-
Reunala T, Lokki J (1978) Dermatitis herpetiformis in Finland. Acta Derm Venereol 58: 505-510.
-
Moi H (1984) Incidence and prevalence of dermatitis herpetiformis in a country in central Sweden, with comments on the course of the disease and IgA deposits as diagnostic criterion. Acta Derm Venereol 64: 144-150.
-
Smith JB, Tulloch JE, Meyer LJ, Zone JJ (1992) The incidence and prevalence of dermatitis herpetiformis in Utah. Arch Dermatol 128: 1608-1610.
-
Ohata C, Ishii N, Hamada T, Shimomura Y, Niizeki H, et al. (2012) Distinct characteristics in japanese dermatitis herpetiformis: A review of all 91 japanese patients over the last 35 years. Clin Dev Immunol 2012: 562168.
-
Ohata C, Ishii N, Niizeki H, Shimomura Y, Furumura M, et al. (2016) Unique characteristics in Japanese dermatitis herpetiformis. Br J Dermatol 174: 180-183.
-
Reunala TL (2001) Dermatitis herpetiformis. Clin Dermatol 19: 728-736.
-
Alonso-Llamazares, J, Gibson, LE, Rogers RS 3rd (2007) Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: Review of the mayo clinic experience. Int J Dermatol 46: 910-919.
-
Karpati S (2012) Dermatitis herpetiformis. Clin Dermatol 30: 56-59.
-
Collin P, Reunala T (2003) Recognition and management of the cutaneous manifestations of celiac disease: a guide for dermatologists. Am J Clin Dermatol 4: 13-20.
-
Karpati S (2004) Dermatitis herpetiformis: close to unravelling a disease. J Dermatol Sci 34: 83-90.
-
Hervonen K, Hakanen M, Kaukinen K, Collin P, Reunala T (2002) First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand J Gastroenterol 37: 51-55.
-
Marietta EV, Camilleri MJ, Castro LA, Krause PK, Pittelkow MR, et al. (2008) Transglutaminase autoantibodies in dermatitis herpetiformis and celiac sprue. J Invest Dermatol 128: 332-335.
-
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140-156.
-
Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N (2002) Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 195: 747-757.
-
Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, et al. (2004) In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 53: 641-648.
-
Troncone R, Jabri B (2011) Coeliac disease and gluten sensitivity. J Intern Med 269: 582-590.
-
Zone JJ, Egan CA, Taylor TB, Meyer LJ (2004) Iga autoimmune disorders: Development of a passive transfer mouse model. J Investig Dermatol Symp Proc 9: 47-51.
-
Hull CM, Liddle M, Hansen N, Meyer LJ, Schmidt L, et al. (2008) Elevation of iga anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 159: 120-124.
-
Zone JJ, Schmidt LA, Taylor TB, Hull CM, Sotiriou MC, et al. (2011) Dermatitis herpetiformis sera or goat anti-transglutaminase-3 transferred to human skin-grafted mice mimics dermatitis herpetiformis immunopathology. J Immunol 186: 4474-4480.
-
Antiga E, Quaglino P, Pierini I, Volpi W, Lami G, et al. (2015) Regulatory t cells as well as il-10 are reduced in the skin of patients with dermatitis herpetiformis. J Dermatol Sci 77: 54-62.
-
Caproni M, Feliciani C, Fuligni A, Salvatore E, Atani L, et al. (1998) Th2-like cytokine activity in dermatitis herpetiformis. Br J Dermatol 138: 242-247.
-
Airola K, Vaalamo M, Reunala T, Saarialho-Kere UK (1995) Enhanced expression of interstitial collagenase, stromelysin-1, and urokinase plasminogen activator in lesions of dermatitis herpetiformis. J Invest Dermatol 105: 184-189.
-
Salmela MT, Pender SL, Reunala T, MacDonald T, Saarialho-Kere U (2001) Parallel expression of macrophage metalloelastase (MMP-12) in duodenal and skin lesions of patients with dermatitis herpetiformis. Gut 48: 496-502.
-
Di Sabatino A, Corazza GR (2009) Coeliac disease. Lancet 373: 1480-1493.
-
Cummins AG, Roberts-Thomson IC (2009) Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol 24: 1347-1351.
-
Lahteenoja H, Irjala K, Viander M, Vainio E, Toivanen A, et al. (1998) Oral mucosa is frequently affected in patients with dermatitis herpetiformis. Arch Dermatol 134: 756-758.
-
Ko CJ, Colegio OR, Moss JE, McNiff JM (2010) Fibrillar IgA deposition in dermatitis herpetiformis--an underreported pattern with potential clinical significance. J Cutan Pathol 37: 475-477.
-
Zone JJ, Meyer LJ, Petersen MJ (1996) Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol 132: 912-918.
-
Barnadas MA (2016) Dermatitis Herpetiformis: A Review of Direct Immunofluorescence Findings. Am J Dermatopathol 38: 283-288.
-
Rose C, Armbruster FP, Ruppert J, Igl BW, Zillikens D, et al. (2009) Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J Am Acad Dermatol 61: 39-43.
-
Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, et al. (2009) Iga anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol 129: 2728-2730.
-
Fry L, McMinn RM, Cowan JD, Hoffbrand AV (1968) Effect of gluten-free diet on dermatological, intestinal, and haematological manifestations of dermatitis herpetiformis. Lancet 1: 557-561.
-
Karell K, Korponay-Szabo I, Szalai Z, Holopainen P, Mustalahti K, et al. (2002) Genetic dissection between coeliac disease and dermatitis herpetiformis in sib pairs. Ann Hum Genet 66: 387-392.
-
Hervonen K, Karell K, Holopainen P, Collin P, Partanen J, et al. (2000) Concordance of dermatitis herpetiformis and celiac disease in monozygous twins. J Invest Dermatol 115: 990-993.
-
Stockbrugger R, Andersson H, Gillberg R, Kastrup W, Lundquist G, et al. (1976) Auto-immune atrophic gastritis in patient with dermatitis herpetiformis. Acta Derm Venereol 56: 111-113.
-
Corazza GR, Di Stefano M, Maurińo E, Bai JC (2005) Bones in coeliac disease: diagnosis and treatment. Best Pract Res Clin Gastroenterol 19: 453-465.
-
Green PH, Jabri B (2002) Celiac disease and other precursors to small-bowel malignancy. Gastroenterol Clin North Am 31: 625-639.
-
Leonard JN, Tucker WF, Fry JS, Coulter CA, Boylston AW, et al. (1983) Increased incidence of malignancy in dermatitis herpetiformis. Br Med J (Clin Res Ed) 286: 16-18.
-
Collin P, Pukkala E, Reunala T (1996) Malignancy and survival in dermatitis herpetiformis: a comparison with coeliac disease. Gut 38: 528-530.
-
Gaspari AA, Huang CM, Davey RJ, Bondy C, Lawley TJ, et al. (1990) Prevalence of thyroid abnormalities in patients with dermatitis herpetiformis and in control subjects with hla-b8/-dr3. Am J Med 88: 145-150.
-
Kaplan RP, Callen JP (1991) Dermatitis herpetiformis: autoimmune disease associations. Clin Dermatol 9: 347-360.
-
Hervonen K, Viljamaa M, Collin P, Knip M, Reunala T (2004) The occurrence of type 1 diabetes in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol 150: 136-138.
-
Neuhausen SL, Steele L, Ryan S, Mousavi M, Pinto M, et al. (2008) Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J Autoimmun 31: 160-165.
-
Stavropoulos PG, Moustou AE, Tsiougou M, Avgerinou G, Chatziolou E, et al. (2008) Might dermatitis herpetiformis be associated with discoid lupus erythematosus? J Eur Acad Dermatol Venereol 22: 749-750.
-
Reunala T, Salmi J, Karvonen J (1987) Dermatitis herpetiformis and celiac disease associated with Addison's disease. Arch Dermatol 123: 930-932.
-
Amato L, Gallerani I, Fuligni A, Mei S, Fabbri P (2000) Dermatitis herpetiformis and vitiligo: report of a case and review of the literature. J Dermatol 27: 462-466.
-
Karabudak O, Dogan B, Yildirim S, Harmanyeri Y, Anadolu-Brasie R (2007) Dermatitis herpetiformis and vitiligo. J Chin Med Assoc 70: 504-506.
-
Bushara KO (2005) Neurologic presentation of celiac disease. Gastroenterology 128: S92-97.
-
Wills AJ, Turner B, Lock RJ, Johnston SL, Unsworth DJ, et al. (2002) Dermatitis herpetiformis and neurological dysfunction. J Neurol Neurosurg Psychiatry 72: 259-261.
-
Aziz I, Hadjivassiliou M, Sanders DS (2015) The spectrum of noncoeliac gluten sensitivity. Nat Rev Gastroenterol Hepatol 12: 516-526.
-
Bonciolini V, Bianchi B, Del Bianco E, Verdelli A, Caproni M (2015) Cutaneous manifestations of non-celiac gluten sensitivity: Clinical histological and immunopathological features. Nutrients 7: 7798-7805.
-
Garioch JJ, Lewis HM, Sargent SA, Leonard JN, Fry L (1994) 25 years' experience of a gluten-free diet in the treatment of dermatitis herpetiformis. Br J Dermatol 131: 541-545.
-
Lewis HM, Renaula TL, Garioch JJ, Leonard JN, Fry JS, et al. (1996) Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br J Dermatol 135: 363-367.
-
Turchin I, Barankin B (2005) Dermatitis herpetiformis and gluten-free diet. Dermatol Online J 11: 6.
-
Janatuinen EK, Pikkarainen PH, Kemppainen TA, Kosma VM, Järvinen RM, et al. (1995) A comparison of diets with and without oats in adults with celiac disease. N Engl J Med 333: 1033-1037.
-
Hardman CM, Garioch JJ, Leonard JN, Thomas HJ, Walker MM, et al. (1997) Absence of toxicity of oats in patients with dermatitis herpetiformis. N Engl J Med 337: 1884-1887.
-
Fry L (2002) Dermatitis herpetiformis: problems, progress and prospects. Eur J Dermatol 12: 523-531.
-
Tjon JM, van Bergen J, Koning F (2010) Celiac disease: how complicated can it get? Immunogenetics 62: 641-651.
-
Zhu YI, Stiller MJ (2001) Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 45: 420-434.
-
Booth SA, Moody CE, Dahl MV, Herron MJ, Nelson RD (1992) Dapsone suppresses integrin-mediated neutrophil adherence function. J Invest Dermatol 98: 135-140.
-
McFadden JP, Leonard JN, Powles AV, Rutman AJ, Fry L (1989) Sulphamethoxypyridazine for dermatitis herpetiformis, linear IgA disease and cicatricial pemphigoid. Br J Dermatol 121: 759-762.
-
Willsteed E, Lee M, Wong LC, Cooper A (2005) Sulfasalazine and dermatitis herpetiformis. Australas J Dermatol 46: 101-103.
-
Hervonen K, Alakoski A, Salmi TT, Helakorpi S, Kautiainen H, et al. (2012) Reduced mortality in dermatitis herpetiformis: a population-based study of 476 patients. Br J Dermatol 167: 1331-1337.
-
Biagi F, Marchese A, Ferretti F, Ciccocioppo R, Schiepatti A, et al. (2014) A multicentre case control study on complicated coeliac disease: Two different patterns of natural history, two different prognoses. BMC Gastroenterol 14: 139.
-
Hervonen K, Salmi TT, Ilus T, Paasikivi K, Vornanen M, et al. (2016) Dermatitis Herpetiformis Refractory to Gluten-free Dietary Treatment. Acta Derm Venereol 96: 82-86.
-
Paek SY, Steinberg SM, Katz SI (2011) Remission in dermatitis herpetiformis: a cohort study. Arch Dermatol 147: 301-305.
-
Herrero-González JE (2010) Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis. Actas Dermosifiliogr 101: 820-826.
-
Ingen-Housz-Oro S, Joly P, Bernard P, Bedane C, Prost C (2011) Dermatitis herpetiformis. Guidelines for the diagnosis and treatment. Centres de reference des maladies bulleuses auto-immunes. Societe francaise de dermatologie. Ann Dermatol Venereol 138: 271-273.
-
Nieuwenhuis WP, Kneepkens CM, Houwen RH, de Beer HJ, Mulder CJ, et al. (2010) Guideline 'Coeliac disease and dermatitis herpetiformis'. Ned Tijdschr Geneeskd 154: A1904.





