Journal of Dermatology Research and Therapy
A Single-Site, Investigator Initiated Open-Label Trial of H.P. Acthar® Gel (Repository Corticotropin Injection) an Adrenocorticotropic Hormone (ACTH) Analogue in Subjects with Moderately to Severely Active Psoriatic Arthritis (PsA)
Justus J Fiechtner*, Tresa Montroy and Joshua June
Colleges of Human and Osteopathic Medicine, Michigan State University, USA
*Corresponding author:
Justus J Fiechtner, MD, MPH, Colleges of Human and Osteopathic Medicine, Michigan State University, 3394 E. Jolly Road, Suite C, Lansing, MI 48910, USA, Tel: 517-272-9727, Fax: 517-853-8238, E-mail: jjfiechtner@gmail.com
J Dermatol Res Ther, JDRT-2-037, (Volume 2, Issue 5), Original Article; ISSN: 2469-5750
Received: September 08, 2016 | Accepted: October 24, 2016 | Published: October 26, 2016
Citation: Fiechtner JJ, Montroy T, June J (2016) A Single-Site, Investigator Initiated Open-Label Trial of H.P. Acthar® Gel (Repository Corticotropin Injection) an Adrenocorticotropic Hormone (ACTH) Analogue in Subjects with Moderately to Severely Active Psoriatic Arthritis (PsA). J Dermatol Res Ther 2:037. 10.23937/2469-5750/1510037
Copyright: © 2016 Fiechtner JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Although several therapeutic agents are available for psoriatic arthritis (PsA), each has limitations. Acthar® Gel (repository corticotropin injection) is FDA-approved for treatment of acute episodes or exacerbation of PsA, but no clinical trials have investigated it for those uses. This 28-week, prospective, single-center, open-label trial included subjects with moderately to severely active PsA. The primary outcome measure was ACR20 at Week 12. Secondary outcomes included ACR20 at Week 24, ACR50, ACR70, Clinical Disease Activity Index (CDAI), Psoriasis Area and Severity Index (PASI) 50, PASI 75, Physician Global Assessment, Patient Global Assessment, Tender and Swollen Joint Count, Visual Analog Scale (VAS) for measurement of pain, acute erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and pre-injection and post-injection cortisol levels at Weeks 12 and 24. Safety, including adverse events and vital signs, were monitored throughout. Among subjects initially enrolled (n = 15), 8 completed Week 12; 7 finished Week 24. All 8 achieved ACR20 at Week 12. At Weeks 12 and 24 there were significant improvements in CDAI, Physician Global Assessment, Patient Global Assessment, Tender and Swollen Joint Count, and VAS (all P ≤ 0.001). At Week 12, 87.5% of subjects reached ACR50, ACR70, PASI50, and PASI75, and 100% did at Week 24. There were no differences in pre-injection or post-injection cortisol levels, ESR, or CRP. Three subjects withdrew early (2 worse psoriasis, 1 depression), but no other adverse events were observed. The remaining 4 patients withdrew for logistical reasons only.
Keywords
ACR20, Adrenocorticotropic hormone, Pain, Physician global assessment, Patient global assessment, Psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is a chronic disease that induces inflammation of the synovial tissue, entheses, and skin [1]. The afflicted subjects have heterogeneous clinical presentations that include diverse articular and dermatological traits, as well as joint and spine complications [1,2]. The comorbidities that impact individuals with PsA include cardiovascular disease, hypercholesterolemia, obesity, hypertension, type 2 diabetes mellitus, anxiety and/or depression, and kidney disease [3]. The subjects also suffer from increased mortality, along with declines in their quality of life [1].
There are several treatment options for PsA available and others currently in clinical trials, including nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), anti-tumor necrosis factor (TNF), anti-interleukin (IL)-17, anti-IL-12/IL-23 and anti-T cell activation medications [1] (Table 1). However, each of the current treatment options has certain limitations. There is a lack of evidence regarding the efficacy of corticosteroids in PsA, and they carry a risk of severe adverse events (AEs) and relapse of skin psoriasis when they are discontinued [1]. The efficacy and safety of some DMARDs for the treatment of PsA are still controversial, and currently it is difficult to quantify the occurrence of AEs during treatment with anti-TNF agents [1].
![]()
Table 1: Efficacy of Acthar Gel for treatment of psoriatic arthritis [DRAFT_1_5MAY2015_v2].
View Table 1
H.P. Acthar® Gel (repository corticotropin injection; Mallinckrodt Brand Pharmaceuticals, Hazelwood, MO), is a highly purified preparation of adrenocorticotropic hormone (ACTH) [4]. Acthar Gel contains the melanocortin peptide ACTH, and it may have mechanisms of action that are consistent with the broader melanocortin system, including anti-inflammatory and immunomodulatory effects [5,6] (Table 2). The 5 melanocortin receptors (MCRs) are located throughout the skin, joint and bone structures [7] (Table 2) that are affected by inflammatory arthritis [6,8] (Table 1 and Table 2).
Currently, Acthar Gel has nineteen different treatment indications designated by the FDA including PsA. Although Acthar Gel is FDA-approved as an adjunctive therapy for short-term administration for either an acute episode or exacerbation of PsA [4], no clinical trials have investigated it for those uses. As current treatment options are not completely satisfactory, clinicians need other potential options. As Acthar Gel has a mechanism of action (MOA) that specifically targets skin, joints and bone, the current study was undertaken. This is the first study designed to assess the efficacy and safety of Acthar Gel among subjects with moderately to severely active PsA who had not responded adequately to prior treatments.
Materials and Methods
Study design
This 28-week, prospective, single-center, open-label trial was conducted between June 24, 2013 and April 30, 2015. All of the subjects were recruited from a single site in Lansing, MI, provided written informed consent, and were free to discontinue treatment at any time. The dose of 80 units twice weekly was based upon the authors' previous experience with a six-month lupus trial conducted at our site and manufacturer's recommendations [9]. The study protocol was approved by an Investigational Research Board/Independent Human Research Ethics Committee and carried out in accordance with Good Clinical Practice guidelines. The study was conducted in accordance with the Declaration of Helsinki 1975, revised Hong Kong 1989. The clinical trial number is NCT01939132.
Inclusion criteria
In order to be included in this trial, subjects had to be 18-75 years of age at the time of screening. Subjects had to meet ACR criteria for PsA and have moderately to severely active PsA that resulted in them either requiring an increase in steroid dose or receiving an add-on steroid despite standard of care therapy. Subjects also had to have been diagnosed with PsA according to the Classification of Psoriatic Arthritis (CASPAR) study group criteria ≥ 6 months prior to screening, and had ≥ 6 tender and ≥ 6 swollen joint counts, and had ≥ 30 minutes of morning joint stiffness.
Subjects who were receiving NSAIDs must have been on stable doses for ≥ 4 weeks prior to initiation of Acthar Gel, and those receiving any DMARDs or biologic agents had to have been treated for ≥ 8 weeks. Doses of oral corticosteroids must have been stable for ≥ 2 weeks prior to initiation of Acthar Gel and not exceed the equivalent of 10 mg of prednisone per day. Subjects had to test negative for tuberculosis within 6 months prior to initiation of Acthar Gel, and females of childbearing potential must have used an effective method of birth control from screening through 60 days after the final dose. Written informed consent and any locally required authorization (e.g., HIPAA) were obtained from each subject prior to performing any protocol-related procedures, including screening evaluations.
Exclusion criteria
Subjects were not permitted to participate if they had any planned surgical intervention between Week 0 (baseline) and Week 24. Individuals were also excluded if they had a known history of a primary immunodeficiency or an underlying condition such as human immunodeficiency virus (HIV) infection that results in an immunocompromised state, sensitivity to proteins of porcine origin, or any concurrent medical conditions or uncontrolled clinically significant systemic diseases, including renal failure, congestive heart failure, hypertension, primary adrenocortical insufficiency or adrenocortical hyperfunction, or peptic ulcer.
Outcome measures
The primary outcome measure in this study was ACR20 at Week 12. The secondary outcome measures included ACR20 at Week 24 and the following assessments at Week 12 and Week 24: Clinical Disease Activity Index (CDAI), Psoriasis Area and Severity Index (PASI), including the PASI50 to determine if subjects achieve 50% improvements, Physician Global Assessment, Patient Global Assessment, Tender Swollen Joint Count, and Visual Analog Scale (VAS) for measurement of pain by subjects. (Physician Global Assessments and PASI scores were determined and measured by the same investigator. Joint counts and Patient Global Assessments were assessed by a designated and different investigator). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were measured at the Week 28 follow-up visit instead of Week 24. Additional calculations of ACR50, ACR70, and PASI75 were also conducted. At Week 0, Week 12, and Week 24 blood samples were obtained prior to and 1 hour after administration of Acthar Gel to measure cortisol levels. Safety, including any adverse events regardless of whether or not they may have been treatment related, and vital signs were monitored throughout the study.
Treatment
Acthar Gel was administered from Week 0 through Week 24 via an aseptic injection technique in which a single dose of 80 units/1 mL was delivered subcutaneously twice weekly within a minimum of 2 days but not more than 4 days from the last injection. The first dose of Acthar Gel was administered at the investigator's site, and during that visit the subjects were educated on aseptic subcutaneous injection technique to allow them to perform home injections. Subjects received injections at the clinic at Week 0, Week 12, and Week 24 so that cortisol levels could be measured, and conducted all other injections themselves. Subjects recorded home injections in a dosing diary that they returned to the study site at each on-site visit.
Data analysis
All numerically continuous data were summarized using means and standard deviations, and P-values for change from Week 0 were calculated based on sample paired t-tests. The P-values ≤ 0.05 were considered statistically significant. No Bonferroni corrections for simultaneous multiple inferences were performed. All analyses were performed using SAS® 9.2 (SAS Institute, Cary, NC).
Results
The mean age of the subjects (n = 15) enrolled in this study was 47.9 years, mean duration of PsA was 8.4 years, and 10 were female [PsAACTH13- Patient Demographic and Therapy Sheet, p1-3]. Previous medications for PsA (mean = 3.8), all current treatments (mean = 2.07), and systemic steroids are presented in table 2 [PsAACTH-13 Current and Previous medications, p1-2].
Eight of the 15 subjects completed treatment through Week 12, and 7 completed treatment through Week 24 [PsAACTH13- Patient Demographic and Therapy Sheet 4-22-15, p1-3]. Among the 7 subjects who withdrew prior to Week 12, 3 had potential treatment-related adverse events (see Safety section below), 1 was lost to follow-up, 1 missed 2 weeks of dosing and was then lost to follow-up, 1 withdrew due to travel commitments, and 1 was noncompliant due to work schedule [PsAACTH13- Patient Demographic and Therapy Sheet 4-22-15, p1-3].
All 8 of the subjects achieved the primary outcome measure of ACR20 at Week 12. Specifically, each of the subjects benefited from decreases in Physician Global Assessment, Patient Global Assessment, CDAI, Tender Joint Count, Swollen Joint Count, and VAS, which comprised all of the clinical measurements (Table 3) [Revised PsAACTH13 DATA 5-4-15, table 2; PsAACTH13 CDAI DATA; Revised PsAACTH13 DATA 5-4-15 table 1]. None of the subjects had scores of 0, which indicate the absence of signs and/or symptoms on each of the scales, on any of those measurements at Week 0. At Week 12 and Week 24, all of them but 1 reached the score of 0 on at least 1 of the scales, and 2 scored 0 on all of the scales. Five subjects reached 0 on the Physician Global Assessment, as did 4 on the Patient Global Assessment, Swollen Joint Count, and VAS [Revised PsAACTH13 DATA 5-4-15, table 2; PsAACTH13 CDAI DATA; Revised PsAACTH13 DATA 5-4-15, table 1].
In addition to all 8 subjects achieving ACR20 at Week 12 (Figure 1), 7 of them (87.5%) also achieved ACR50, ACR70, PASI50, and PASI75 at Week 12. All 7 of the subjects who completed the entire study achieved each of those endpoints at Week 24 (Figure 1).
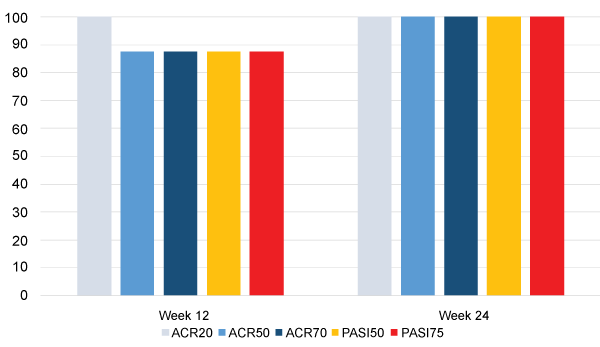
.
Figure 1: Percentage of subjects reaching ACR20, ACR50, ACR70, PASI50, and PASI75.
View Figure 1
As shown in table 1, the improvements from Week 0 to Week 12 and Week 24 in Physician Global Assessment, Patient Global Assessment, CDAI, Tender Joint Count, Swollen Joint Count, and VAS were statistically significant (all P-values ≤ 0.001) [DRAFT_1_5MAY2015_v2]. There were no significant changes in ESR or CRP. There were no significant within-subject differences between pre-injection cortisol levels at Week 0, Week 12, and Week 24. There were also no significant differences between the post-injection levels at those time points. However, there were significant within-subject differences between pre-injection and post-injection cortisol levels at Weeks 0, 12, and 24 (P-value < 0.0001; table 1) [DRAFT_1_5MAY2015_v2; Revised PsAACTH13 DATA 5-4-15, table 2].
![]()
Table 2: Steroids and current and previous medications [PsAACTH13 Current and Previous Medications].
View Table 2
The PASI scores obtained from each of the subjects are presented in table 4 [Revised PsAACTH13 DATA 5-4-15, table 3]. Only subject 12 failed to improve in PASI from Week 0 to Week 12. At every other interval the PASI scores of all of the other subjects either improved or remained unchanged, especially since the majority of them were 0.
![]()
Table 3: Lab results and clinical assessments [Revised PsAACTH13 DATA 5-4-15, table 2].
View Table 3
![]()
Table 4: Psoriasis area and severity index [Revised PsAACTH13 DATA 5-4-15, table 3].
View Table 4
Safety
There were no changes in blood pressure, body temperature, or blood glucose levels observed among any of the subjects throughout this study. Only 3 subjects left the study for reasons that were possibly treatment related (2 had worse psoriasis and 1 had depression) [PsAACTH13- Patient Demographic and Therapy Sheet 4-22-15, p1-3; PSAACTH13 Revised Subject Data, p3-4]. Although one of those 2 individuals with psoriasis left after 8 doses of Acthar Gel and the other after 6, both of those departures occurred during Week 4. The subject with depression left after Week 2. No skin flares were reported by any of the other subjects or observed by the investigators prior to Week 24 or between Week 24 when the last dose of Acthar Gel was administered and the end of the 30 day follow-up period at Week 28. There were no other potentially treatment-related adverse events identified throughout this study.
Discussion
This is the first trial designed to assess the efficacy and safety of Acthar Gel for the treatment of PsA, and the results clearly demonstrate substantial improvements among the subjects who completed the study. All of the subjects met ACR criteria for moderately to severely active PsA and therefore required additional DMARDs or add-on steroid therapy, and they had not responded adequately to at least 1and as many as 6 other treatments prior to this trial.
A rigorous methodology was utilized for measuring the efficacy of Acthar Gel in this study, and each assessment indicated improvements in multiple parameters used to measure quality of life and daily overall function of subjects, including improvement in joint function and reduction in pain. An important aspect to the study design is that it provides both physician-reported and subject-reported assessments. There were statistically significant improvements in both types of outcome measures demonstrating consistency between physician and subject evaluation of treatment results, further validating the findings. Clinical benefits were apparent in the primary outcome measure of ACR20, as well as the higher threshold ACR50 and ACR70 and the PASI50 and PASI75.
While the results of this trial indicate Acthar Gel can improve clinical aspects of PsA, the mechanism of action is under investigation. Importantly, a study that utilized a mouse model found that the melanocortin neuropeptide α-melanocyte-stimulating hormone (α-MSH), which is a mediator of skin pigmentation,can suppress activation and proliferation of effector T cells among models with psoriasis [10]. This indicates that immunosuppressive regulatory T cells that are activated by α-MSH can inhibit progression of psoriasis-like skin inflammation and thereby suppress both activation and proliferation of effector T cells [10]. This could be particularly relevant since α-MSH and ACTH are both products of the same protein molecule [8].
The MOA of Acthar Gel was historically attributed to stimulation of adrenal cortisol secretion. However, data has suggested that the MOA cannot be explained by steroidogenic activity alone [5,6] (Table 2). Acthar Gel contains melanocortin peptides, such as ACTH and may have MOAs that are consistent with the broader melanocortin system, including anti-inflammatory and immunomodulatory effects [5,6] (Table 2). The 5 melanocortin receptors (MCRs) are among the G protein-coupled receptor category that are located throughout the human anatomy, including the central nervous system and immune cells [6,8] (Table 2). The MCRs are also located throughout the skin and bone structures [7] (Table 2) that are afflicted by inflammatory arthritis [6,8] (Table 1 and Table 2) and ACTH has been shown to bind to each of those MCRs with high affinity even though they differ in selectivity for various melanocortin peptides [6,8] (Table 2).
These results will need to be confirmed in additional studies given the small number of subjects included in this trial, as well as the fact that they were all recruited from one location and there were no active comparator or placebo treated patients. The patients in this study were all women and not necessarily representative of the general population of PsA subjects, which consists of an equal percentage/proportionof men and women [11]. This study population does, however, represent the average 30-50 year old age at onset of PsA. Three patients experienced adverse events possibly related to the treatment medication, including skin flare. It is also important to note that although there were significant increases in cortisol levels 1 hour after the administration of Acthar Gel, those increases subsided prior to the time when the next blood sample was acquired at the clinic. In fact, at Week 12 and Week 24 the pre-injection cortisol levels did not differ significantly from the pre-injection levels at Week 0, indicating the levels returned to normal. This suggests that adrenal function with regard to cortisol production was not impaired during the Acthar Gel treatment regimen. In contrast, patients who are receiving prednisone at higher doses and with increased duration of treatment might be expected to show some evidence of adrenal insufficiency [12]. However, further research, including larger populations, is obviously needed before any conclusions can be made.
Acthar Gel is approved by the FDA for treatment of an acute episode or exacerbation of PsA, but this is the first study designed to measure the efficacy and safety of that drug among such subjects. The results of this PsA trial reveal that treatment with Acthar Gel resulted in clinical benefits that were appreciated by physicians as well as patients previously with inadequate responses to multiple treatments, as well as the standard measures of ACR 20, 50, and 70, and PASI scores.
Disclosures
J.F.has received grant/research support from Autoimmune and Rare Diseases Business (formerly Questcor) Mallinckrodt Pharmaceuticals.
T.M. has received grant/research support from Autoimmune and Rare Diseases Business (formerly Questcor) Mallinckrodt Pharmaceuticals.
J.J. has received grant/research support from Autoimmune and Rare Diseases Business (formerly Questcor) Mallinckrodt Pharmaceuticals.
Conflicts of Interest
Funding to support the preparation of this manuscript was originally provided by Questcor Pharmaceuticals, Inc., and continued by Mallinckrodt Pharmaceuticals to MedVal Scientific Information Services, LLC, Skillman, NJ.
Acknowledgments
The author thanks Aric Fader, PhD, of MedVal Scientific Information Services, LLC, for providing professional writing and editorial assistance. Funding to support the preparation of this manuscript was originally provided to MedVal by Questcor Pharmaceuticals, Inc., and continued by Mallinckrodt Pharmaceuticals. This manuscript was prepared according to the International Society for Medical Publication Professionals' "Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines" and the International Committee of Medical Journal Editors' "Uniform Requirements for Manuscripts Submitted to Biomedical Journals".
References
-
Liu JT, Yeh HM, Liu SY, Chen KT (2014) Psoriatic arthritis: Epidemiology, diagnosis, and treatment. World J Orthop 5: 537-543.
-
Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, et al. (2008) Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 58: 851-864.
-
Khraishi M, Aslanov R, Rampakakis E, Pollock C, Sampalis JS (2014) Prevalence of cardiovascular risk factors in patients with psoriatic arthritis. Clin Rheumatol 33: 1495-1500.
-
(2015) H.P. Acthar® Gel (repository corticotropin injection) [prescribing information]. Mallinckrodt ARD Inc, Hazelwood, MO, USA.
-
Levine T (2012) Treating refractory dermatomyositis or polymyositis with adrenocorticotropic hormone gel: a retrospective case series. Drug Design Devel Ther 6: 133-139.
-
Catania A, Gatti S, Colombo G, Lipton JM (2004) Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56: 1-29.
-
Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M (2013) Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler J 19: 130-136.
-
Brzoska T, Luger TA, Maaser C, Abels C, Bohm M (2008) Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29: 581-602.
-
Fiechtner J, Montroy T (2016) Six months' treatment of moderately to severely active systemic lupus erythematosus with repository corticotropin injection: an extension of a single-site, open-label trial.
-
Auriemma M, Brzoska T, Klenner L, Kupas V, Goerge T, et al. (2012) α-MSH-stimulated tolerogenic dentritic cells induce functional regulatory T cells and ameliorate ongoing skin inflamnmation. J Investig Dermatol 132: 1814-1824.
-
http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Psoriatic_Arthritis/
-
Sacre K, Dehoux M, Chauveheid MP, Chauchard M, Lidove O, et al. (2013) Pituitary-adrenal function after prolonged glucocorticoid therapy for systemic inflammatory disorders: an observational study. J Clin Endocrinol Metab 98: 3199-3205.





