Journal of Dermatology Research and Therapy
Pyoderma Gangrenosum: A Review of Orthopedic Case Reports
Stephanie Chapman1, Daniel Delgadillo III1 and David T. Rispler2*
1Michigan State University College of Human Medicine, Grand Rapids, MI, USA
2Orthopedic Residency Program, Grand Rapids Medical Education Partners, Grand Rapids, MI, USA
*Corresponding author:
David T. Rispler, MD, Grand Rapids Orthopedic Residency Program, 200 Jefferson Ave SE, Jefferson Building Suite 305, Grand Rapids, MI 49503, USA, Tel: 616-685-6615, 616-890-5658, Fax: 616-685-3033, E-mail: drispler@comcast.net
J Dermatol Res Ther, JDRT-2-018, (Volume 2, Issue 2), Short Review; ISSN: 2469-5750
Received: February 11, 2016 | Accepted: March 07, 2016 | Published: March 09, 2016
Citation: Chapman S, Delgadillo D, Rispler DT (2016) Pyoderma Gangrenosum: A Review of Orthopedic Case Reports. J Dermatol Res Ther 2:018. 10.23937/2469-5750/1510018
Copyright: © 2016 Chapman S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pyoderma gangrenosum (PG) is an uncommon disease characterized by necrotic ulcers that are often associated with underlying systemic disease. PG can occur at the surgical site following surgery, including orthopedic surgery, and may be commonly mistaken for postoperative infection, delaying diagnosis and resulting in wound deterioration and subsequent sequelae. Previously, 20 case reports of PG have been reported after orthopedic surgery. We reviewed these cases and found the majority (60%) were women. Forty percent had underlying evidence of systemic disease. Debridement was performed in 70%. Development of satellite lesions led to diagnosis of PG in 35%. These cases underscore the difficulty in diagnosis of PG and the use of inappropriate treatment, thereby highlighting the need for better awareness, diagnostic criteria, and clinical management of this disease.
Keywords
Pyoderma gangrenosum, Ulcers, Case report, Review, Orthopedic surgery, Postoperative infection
Introduction
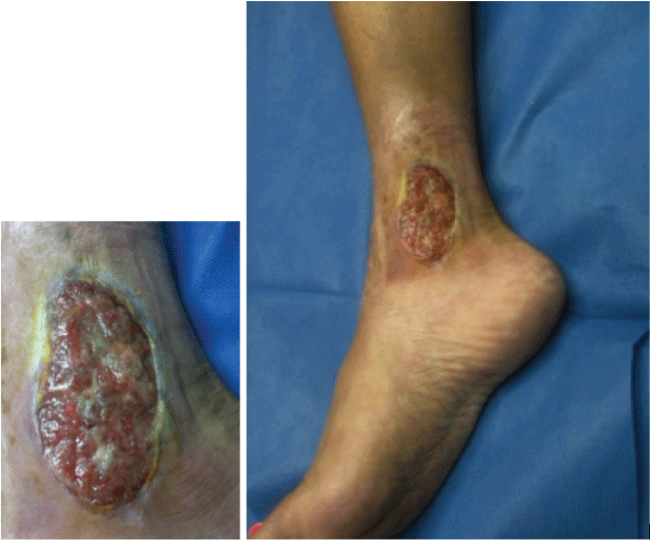
PG was first described by Brocq [1] in 1916 and then by Brunsting et al. [2] in 1930. PG is a noninfectious neutrophilic dermatosis that manifests clinically as papules and pustules that enlarge and become undermined, painful necrotic ulcers with a bluish border and surrounding erythema and edema (Figure 1). The necrotic ulcers must be distinguished from bacterial infection, and this can be challenging as the ulcers may become secondarily infected and develop a purulent exudative cover. The peak incidence of disease is between the ages of 20 to 50 years with women being more often affected than men [3]. It is estimated that the disease affects somewhere between 3 and 10 patients per million [4]. In 50-70% of patients, PG occurs in association with an underlying systemic disease such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA) or seronegative arthritis, and hematologic disorders [3,5,6]. PG can develop in response to various medications or as a pathergic response at sites of skin damage after trauma and surgery [3].
.
Figure 1: PG ulcer with an undermined bluish border and surrounding erythema and edema.
View Figure 1
We present an analysis of cases of PG reported in the orthopedic literature, and a review of the important clinical features of disease. The purpose of this report is to educate more health care providers on the possibility of PG in orthopedic surgery post-operative patients. A greater awareness of this condition may improve diagnostic accuracy and reduce patient morbidity.
Review of Individual Case Reports in the Orthopedic Literature
PG can occur following any kind of surgery including breast, abdominal, and orthopedic surgery [3]. A PubMed search was conducted to find case reports of PG following orthopedic surgery using the search term ‘pyoderma gangrenosum orthopedic surgery’. A total of 20 cases of PG following orthopedic surgery described in the English language were found and are shown in Table 1 [7-25]. Eight cases followed total knee replacement [7-14], 4 followed hip surgery [15-18], 2 after foot and ankle surgery [19,20], 1 after surgery on a rheumatoid hand [19], 1 followed meniscectomy [21], 1 followed patellar tendon repair [22], 1 after achilles tendon reconstruction [23], 1 after a traumatic tibial fracture [24], and 1 after rotator cuff repair [25]. In review, 60% affected women. In both cases of bilateral total knee arthroplasty, PG developed on both knees [8,12]. Forty percent of patients had a significant past medical history including arthritis, myelodysplastic syndrome, and a vague history of wound complication. One patient had a history of both Crohn’s disease and RA and another had a prior history of PG following a dog bite.
![]()
Table 1: Cases of Pyoderma Gangrenosum Following Orthopedic Surgery.
View Table 1
In the postoperative period, PG can easily be confused with wound infection, delaying diagnosis and contributing to significant patient morbidity. Patients will often receive multiple treatments by debridement for suspected wound infection that exacerbate the progression of disease due to pathergy, an aberrant immune response characterized by development of new lesions in response to trauma or surgery [5]. Among the cases of PG following orthopedic surgery, 70% received at least one wound debridement before PG was accurately diagnosed, and 50% received multiple treatments by debridement. The development a satellite lesion distant to the site of surgery aided in the correct diagnosis in 35%. A delay in diagnosis led to a subsequent below-the-knee amputation in one patient due to an inability to reconstruct the affected foot, and a partial toe amputation in another due to compromised blood supply. Systemic steroids were used in the treatment of all cases. Four patients required skin grafts. One patient required a latissimus dorsi myocutaneous flap in addition to a skin graft for reconstruction, while another patient required a free fasciocutaneous anterolateral thigh flap and gastrocnemius flap for closure. One other patient required a free flap and rotational flap for closure of a large shoulder defect. This highlights the ability to perform uncomplicated surgery if accurate diagnosis and appropriate treatment is employed prior to surgery.
Discussion
PG is a neutrophilic dermatosis characterized by papules and pustules that rapidly progress to undermined necrotic ulcerations that can occur following surgery [3,5]. Several clinical variants of PG have been described in the literature: ulcerative, pustular, bullous and vegetative [3,5,6]. Ulcerative type classically presents on the legs, although it can occur anywhere and is associated with IBD and arthritis. The evolution of ulcerative PG involves pustules that enlarge and necrose, forming ulcers with a blue, damaged border and surrounding erythema. Pustular type commonly presents on the limbs as discrete, superficial pustules that coalesce and ulcerate, and is commonly associated with IBD. Bullous type typically occurs in association with hematologic malignancy and is characterized by painful vesicles that enlarge into bullae with a necrotic center. Vegetative type typically presents on the trunk with a superficial, solitary ulcer. Vegetative PG is considered to be less aggressive and is usually not associated with an underlying systemic disease [5].
Pathogenesis is multifactorial and thought to involve abnormalities in neutrophil function and inflammatory mediators as well as a genetic predisposition [4]. Defects in neutrophil migration, chemotaxis, and phagocytosis have been reported in patients with PG. Increased activity of neutrophils is thought to contribute to pathergy, an aberrant immune response to skin antigens altered by surgery or trauma responsible for causing PG in about 20-30% of patients. Various pro-inflammatory cytokines and other inflammatory mediators have also been implicated in pathogenesis. Immunohistochemical studies have demonstrated high levels of IL-8, a chemoattractant for neutrophils, in PG ulcers. Studies have also shown that IL-17, IL-23, TNF-alpha, and matrix metalloproteinases are overexpressed in PG lesions and that there is an imbalance of T-regulatory cells and Th17 cells in patients with PG. A number of genetic mutations have been reported, and in some cases a constellation of diseases occur in concert with PG. PAPASH syndrome is characterized by pyogenic arthritis, PG, hidradenitis suppurativa, and acne as a result of a mutation in the PSTPIP-1 gene. Genetic mutations implicated in IBD susceptibility have also been correlated with the development of PG [4].
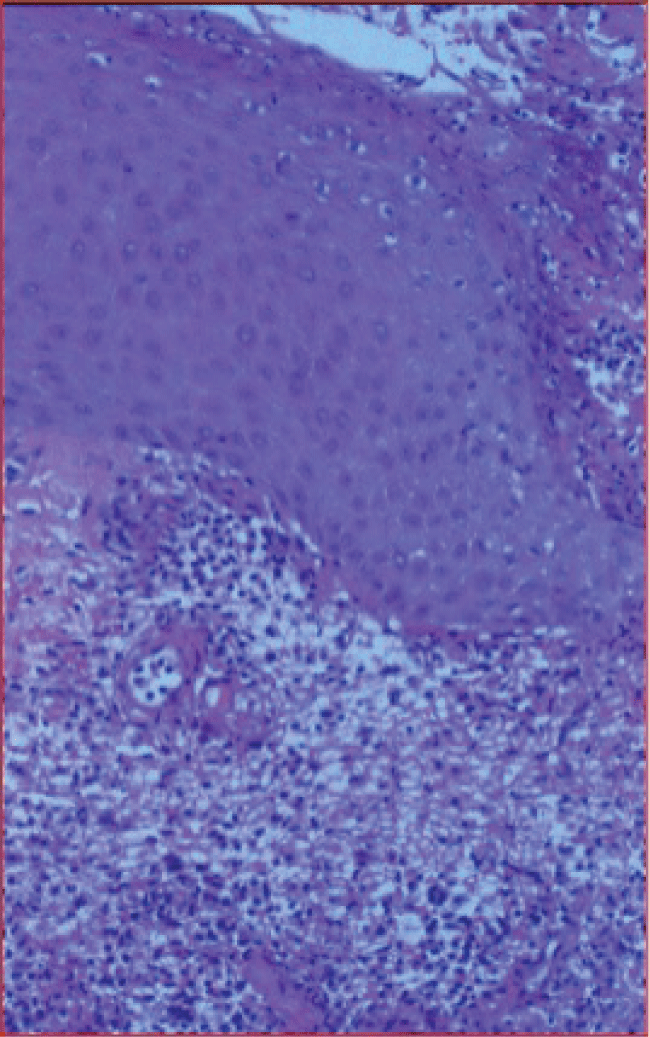
Diagnosis is based predominantly on clinical findings. Obtaining a detailed past medical history of PG or an underlying systemic illness may help lead to the appropriate diagnosis. Diagnosis is made challenging by the fact that there is no laboratory parameter diagnostic of PG and biopsies of skin lesions demonstrate non-specific acute inflammatory changes with a preponderance of neutrophils [3,7] (Figure 2). Additionally, PG may mimic infection. Important clues to the diagnosis include sterile wound cultures, failure of ulcerative lesions to respond to antibiotic therapy, and provocation of disease after wound debridement [7]. In practice, the development of satellite lesions distant to the site of surgery has proven to aid diagnosis. Major and minor diagnostic criteria have been proposed and may serve as a useful tool to the clinician when considered together [26,27]. Major criteria include: 1) a rapidly progressing, painful, and necrolytic cutaneous ulcer, 2) exclusion of other cutaneous diseases. Minor criteria include: 1) a history of pathergy, 2) presence of an underlying systemic disease associated with PG, 3) characteristic histopathologic findings, 4) rapid response to steroids [27].
Approach to treatment involves both local and systemic therapies [5,28]. Local therapy is centered on proper wound care and prevention of secondary infection. Topical corticosteroids may also be used alone or as an adjunct to systemic therapy [6]. Skin grafting is typically unsuccessful and has been associated with the formation of new lesions at the donor site [19]. However, in this review we found surgery was performed successfully after appropriate treatment had been employed. Options for systemic therapy include corticosteroids such as oral prednisone at a dose of 1mg/kg/day until lesions significantly improve followed by a taper. Intravenous pulse administration of high dose methylprednisolone can be used for rapid relief in refractory cases. Other systemic treatment options include TNF-alpha antagonists such as infliximab (which has the highest level of evidence), adalimumab, or etanercept, and calcineurin inhibitors such as cyclosporine, antimicrobials such as dapsone, and chemotherapeutic agents such as cyclophosphamide and methotrexate [19,27].
In conclusion, prompt identification and treatment of PG is imperative to decrease patient morbidity. This comprehensive analysis of individual case reports of PG in the orthopedic literature highlights the challenge of diagnosis in the postoperative period and the need for increased education amongst clinicians. In cases where cutaneous lesions develop postoperatively, PG should be considered in the differential, especially among patients with strong risk factors. Efforts to increase awareness of this disease should be taken to prevent misdiagnosis of infection and aggressive treatment by debridement that only exacerbates this condition.
Acknowledgements
Robert S. Kirsner, MD
Department of Dermatology, University of Miami, Miami, FL, USA
References
-
Brocq L (1916) Nouvelle contribution al’étude du phagédénismegéométrique (New contribution to the study of geometric phagedaenism). AnnDermatolSyphiligr (Paris) 6: 1-39.
-
Brunsting LA, Goeckerman WH, O'Leary PA (1930) Pyoderma (echthyma) gangrenosum: Clinical and experimental observations in five cases occurring in adults. Archives of Dermatology 22: 655-680.
-
Wollina Uwe (2007) Pyoderma gangrenosum- a review. Orphanet J Rare Dis 2: 19.
-
Braswell SF, Kostopoulos TC, Ortega-Loayza AG (2015) Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol 73: 691-698.
-
Powell FC, Su WP, Perry HO (1996) Pyoderma gangrenosum: classification and management. J Am Acad Dermatol 34: 395-409.
-
Brooklyn T, Dunnill G, Probert C (2006) Diagnosis and treatment of pyoderma gangrenosum. BMJ 333: 181-184.
-
Jain A, Nanchahal J, Bunker C (2000) Pyoderma gangrenosum occurring in a lower limb fasciocutaneous flap--a lesson to learn. Br J Plast Surg 53: 437-440.
-
Wadia F, Malik MH, Porter M (2007) Postoperative Wound Breakdown Caused by PyodermaGangrenosum after Bilateral Simultaneous Total Knee Arthroplasty. J Arthroplasty 22: 1232-1235.
-
Nakajima N, Ikeuchi M, Izumi M, Kuriyama M, Nakajima H, et al. (2011) Successful treatment of wound breakdown caused by pyoderma gangrenosum after total knee arthroplasty. Knee 18: 453-455.
-
Hill DS, O’Neill JK, Toms A, Watts AM (2011) Pyodermagangrenosum: A report of a rare complication after knee arthroplasty requiring muscle flap cover supplemented by negative pressure therapy and hyperbaric oxygen. J Plast Reconstr Aesthet Surg 64: 1528-1532.
-
Mandal A, Addison P, Stewart K, Neligan P (2006) Vacuum-assisted closure therapy in pyodermagangrenosum. Eur J Plast Surg 28: 529-531.
-
Attar S, Spangehl M, Casey III WJ, Smith AA (2010) PyodermaGangrenosum Complicating Bilateral Total Knee Arthroplasty. Orthopedics 33.
-
Yik JH, Bigliardi PL, Sebastin SJ, Cabucana NJ, Venkatachalam I, et al. (2015) Pyoderma gangrenosum mimicking early acute infection following total knee arthroplasty. Ann Acad Med Singapore 44: 150-151.
-
Verma SB (2009) Atypical pyodermagangrenosum following total knee replacement surgery: First report in dermatologic literature. An Bras Dermatol 84: 689-691.
-
De Thomasson E, Caux I (2010) Pyodermagangrenosum following an orthopedic surgical procedure. Orthop Traumatol Surg Res 96: 600-602.
-
Suarez C, Mercier E, Piquet P, Montané de la Roque P, Bordes JP, et al. (2011) Comments on: "Pyoderma Gangrenosum following an orthopedic surgical procedure" by E. de Thomasson, and I. Caux, published in Orthop Traumatol Surg Res 2010;96:600-602. Orthop Traumatol Surg Res 97: 890-891.
-
Armstrong PM, Ilyas I, Pandey R, Berendt AR, Conlon CP, et al. (1999) Pyoderma gangrenosum. A diagnosis not to be missed. J Bone Joint Surg Br 81: 893-894.
-
Nizamoglu M (2015) Pyoderma Gangrenosum Mimicking an Infected Wound following Dynamic Hip Screw Fixation. Case Rep Orthop 2015: 571472.
-
Bennett CR, Brage ME, Mass DP (1999) Pyoderma gangrenosum mimicking postoperative infection in the extremities. A report of two cases. J Bone Joint Surg Am 81: 1013-1018.
-
Melo Grollmus R, Fernández de Retana P (2013) Pyoderma gangrenosum following foot and ankle surgery: a case report. Foot Ankle Int 34: 745-748.
-
Madsen JT, Skov O, Andersen KE (2009) Pyoderma gangraenosum as a complication to knee arthroscopy. Knee 16: 299-300.
-
Wanich T, Swanson AN, Wyatt AJ, Kelly AM (2012) Pyodermagangrenosum following patellar tendon repair: a case report and review of the literature. Am J Orthop (Belle Mead NJ) 41: E4-E9.
-
Fang Z, Waizy H, Berger S, Stukenborg-Colsman C, Plaass C (2013) Pyoderma Gangrenosum Following Orthopaedic Surgery. The Journal of Bone and Joint Surgery 3.
-
Steenbrugge F, Raaijmaakers M, Caekebeke P, Van Landuyt K (2011) Pyoderma gangrenosum following trauma of the knee: a case of pathergy and review of orthopaedic cases. Injury 42: 421-423.
-
Reid AB, Stanley P, Grinsell D, Daffy JR (2014) Severe, Steroid-responsive, Myositis Mimicking Necrotizing Fasciitis following Orthopedic Surgery: A Pyoderma Variant with Myonecrosis. Plast Reconstr Surg Glob Open 2.
-
Teagle A, Hargest R (2014) Management of pyoderma gangrenosum. J R Soc Med 107: 228-236.
-
Su WPD, Davis MDP, Weenig RH, Powell FC, Perry HO (2004) Pyodermagangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 43: 790-800.
-
Cohen PR (2009) Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol 10: 301-312.