International Journal of Diabetes and Clinical Research
Diabetes in Rural Rwanda: High Retention and Positive Outcomes after 24 Months of Follow-up in the Setting of Chronic Care Integration
Neo Tapela1,2,3*, Hamissy Habineza1, Sarah Anoke4, Emmanuel Harerimana5, Francis Mutabazi5, Bethany L. Hedt-Gauthier1,3, Symaque Dusabeyezu5, Pie X F. Uwiragiye1, Cadet Mutumbira5, Gedeon Ngoga1, Deogratias Ndagijimana5, Emmanuel Rusingiza5,6, Gene Bukhman1,2,3# and Charlotte Bavuma5,6#
1Partners in Health/Inshuti Mu Buzima, Kacyiru, Kigali, Rwanda
2Brigham & Women's Hospital, USA
3Harvard Medical School, USA
4Harvard T. H. Chan School of Public Health, USA
5Ministry of Health, Rwanda
6University of Rwanda, Rwanda
#Senior authors contributed equally to the manuscript
*Corresponding author:
Neo Tapela, MD, MPH, Botswana Harvard AIDS Institute Partnership (BHP) Private Bag BO 320, Gaborone, Botswana, Tel: +267 7454 7004 (Botswana), +1 617 785 4758 (US), Fax +267 3901284, E-mail: ntapela@gmail.com
Int J Diabetes Clin Res, IJDCR-3-058, (Volume 3, Issue 2), Research Article; ISSN: 2377-3634
Received: January 11, 2016 | Accepted: May 17, 2016 | Published: May 20, 2016
Citation: Tapela N, Habineza H, Anoke S, Harerimana E, Mutabazi F, et al. (2016) Diabetes in Rural Rwanda: High Retention and Positive Outcomes after 24 Months of Follow-up in the Setting of Chronic Care Integration. Int J Diabetes Clin Res 3:058. 10.23937/2377-3634/1410058
Copyright: © 2016 Tapela N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aims: This study describes the baseline characteristics and 24 month outcomes of diabetic patients managed in an integrated chronic care program at public facilities in rural Rwanda.
Methods: Retrospective review of routine electronic medical records of patients treated for diabetes between October 1, 2006 and September 30, 2014 was conducted. Baseline demographic and clinical characteristics are described. Outcomes of HbA1c values, loss to follow-up and death are reported.
Results: Of 544 patients enrolled, 305 (56.1%) were female and twenty-two (4.0%) were younger than 18 years. Of those with adequate documentation, 72.3% were subsistence farmers, 35.8% had baseline BMI ≥ 25, and 5.3% were current smokers. Median HbA1c was 10.3% (IQR: 8.3, 11.9) at baseline and 8.2% (IQR: 6.3, 10.1) at seven to 12 months of follow-up. For 394 patients with at least 24 months between enrollment and study period end, 66 (16.8%) were lost-to-follow-up, 12 (3.0%) died within the first 24 months and 316 (80.2%) were alive and in care.
Conclusions: Our findings indicate that diabetes can be effectively managed with reasonable outcomes in a rural resource-limited setting. We also found relatively low lifestyle risk factors and comorbidities among our patients compared with those in the United States and Europe.
Keywords
Diabetes, Sub-Saharan Africa, Nurse-led care, Task-shifting, Rwanda
Introduction
The increasing burden of chronic non-communicable diseases (NCDs) around the world is particularly concerning in low- and middle-income countries (LMICs), where 80% of NCD-related deaths occur [1]. In 2008, diabetes affected 382 million adults worldwide [2] and accounted for 1.3 million deaths [1]. In sub-Saharan Africa, diabetes is predicted to rise rapidly, increasing by 80% over 20 years and affecting 18.7 million by the year 2025 [3]. Diabetes, however, is not just an emerging problem in this region; it has long been an endemic killer of the rural poor [4]. Diabetes also causes significant morbidity, increasing the risk of non-traumatic lower limb amputations by ten-fold [1], as a leading cause of renal failure [1,5,6] and visual impairment [1] and as a major risk factor for cardiovascular disease around the world [1,7].
Despite this, access to services for diabetes care in sub-Saharan Africa is limited and there are significant disparities in patient outcomes when compared with global statistics. In 2013, although only 8.6% of all deaths in sub-Saharan Africa were attributed to diabetes, 76.4% of these deaths occurred in people under the age of 60 years, compared to the 50% or less among diabetes-related deaths world-wide [2]. Access to services for rural patients is particularly lacking [8]. Contributing factors include few trained personnel, expensive medications and equipment [9], late presentation, delayed diagnosis, as well as treatment complications related to food in security such as hypoglycemia [3].
Although literature provides several examples of diabetes programs in sub-Saharan Africa [6-16], few describe outcomes from programs based in rural settings in low-income countries where care is coordinated between secondary facilities, primary health centers and the community. Since 2006, Partners In Health (PIH)-an international non-governmental organization with a mandate to deliver healthcare to rural poor-has supported the Rwandan Ministry of Health (RMOH) in delivering care for diabetes and other NCDs at public facilities in rural districts through a strategy of progressive chronic care integration [17,18]. More complex outpatient services-such as insulin management and anticoagulation are delivered at district hospitals, by specialized nurses who also provide training, mentorship and supervision to health center staff. Chronic care at health centers is provided in a more integrated fashion, while community health workers contribute to adherence support and case finding for symptomatic patients. Here, we describe the diabetic population served and disease outcomes at these facilities, with a view of informing approaches to diabetes care delivery in similar settings.
Methods
Study setting
Although Rwanda has a gross national income of US$630 per capita (2013) [19] and 70% of the rural population was living in multidimensional poverty in 2010 [20], the RMOH has made much progress in health and development by emphasizing equity and focusing on primary care and integration of services [21]. However, similar to other countries in the region, diabetes remains a significant problem. National records indicate that diabetes was among the top five causes of morbidity in the internal medicine department of a national referral hospital in Butare [22], and a study conducted at Rwanda's national teaching hospital in Kigali found significant morbidity and complications among admitted and ambulatory diabetes patients [15]. Access to diabetes services has been expanded through universal public health insurance coverage and outreach efforts by trained pediatric diabetes clinicians to some district hospitals [23]. However, gaps remain including weak monitoring and evaluation systems nationally [24], limited equipment for glucose and HbA1c testing, general lack of population awareness about diabetes [25] and too few facilities providing longitudinal services accessible to rural-based patients.
In Rwanda, PIH supports delivery of health services at public facilities in three rural districts, with a total catchment of 850,000 people. Southern Kayonza and Kirehe Districts are located in the Eastern Province, while Burera District is located in the Northern Province. NCD programs have been established at the three district hospitals and seven health centers (out of the 41 health centers within these three districts) and, as of June 2015, over 3,000 patients have been enrolled in the NCD program [18].
Patient management
The model of comprehensive chronic care integration employed at PIH-supported public facilities in Rwanda is described in detail elsewhere [17,18,26]. For diabetes, nurses receive didactic and practical training on diagnosis and long-term management of diabetic patients that is complemented by long-term mentorship from physicians and specialist nurses. At the district hospitals, each NCD clinic is staffed with at least two trained RMOH nurses who cover the diabetes-specific clinic held once a week. Patients requiring admission are cared for by physicians and nurses in the pediatrics or internal medicine wards, in consultation by NCD clinic team. At each of the seven health centers with NCD clinics, two trained RMOH nurses take turns covering the weekly NCD clinic that includes diabetes but is not disease-specific. Health center NCD clinics receive visits from a district hospital NCD nurse at least once a month for continued mentorship, employing a structured mentorship model that has been established for women's health, HIV and other clinical spheres [27,28].
Diagnosis and monitoring employs point-of-care testing for urine glucose, urine ketones, blood glucose and, for the district hospitals only, hemoglobin A1c (HbA1c). Additionally, tests routinely available at the facility's laboratory, such as renal function tests, are performed to support diagnosis and monitoring. Treatment includes individual counseling on lifestyle risk factor modification and group patient education sessions, oral hypoglycemic agents (glibenclamide, or metformin if BMI > 25.0), insulin (routinely available at the district hospital but only available for emergencies at the health center level) and socioeconomic supports such as food, transport vouchers and community health worker follow-up for vulnerable patients. Frequency of patient visits for clinical consultation ranges from weekly to every two months, depending on acuity of a patient's illness.
Data collection and analysis
We conducted a retrospective review of routine electronic medical records (EMR) of patients managed for diabetes at PIH-supported public NCD programs between October 1, 2006 and September 30, 2014. Where electronic data were missing or ambiguous, manual chart abstraction was conducted. Patients who met the following criteria were excluded: missing date of enrollment, date of enrollment was prior to October 1, 2006 or later than September 30, 2014, and baseline HbA1c < 6.5.
We report number and frequency of patient demographic and clinical characteristics at baseline. Given the relatively recent (June 2012) establishment of the oncology component of EMR and resultant incomplete data entry, data related to cancer as a comorbidity were not included. We report outcomes of death, loss to follow-up and HbA1c value measured at follow-up milestones of 6 (defined as four to six months follow-up, inclusive), 12 (seven to 12 months follow-up), 18 (13 to 18 months follow-up) and 24 months (19 to 24 months follow-up). Loss to follow-up (LTFU) for a particular milestone was defined as patient's last visit occurring 3 months or more before the end of the milestone period. Data were administratively censored at September 30, 2014 and individuals who did not have enough months of follow-up were excluded from the reported outcomes for that follow-up milestone period. Differences between group HbA1c values at baseline and at 6, 12, 18 and 24 month follow-up milestones were assessed using Wilcoxon sign-rank tests at the α = 0.05 significance level. All analyses were conducted using Stata v12 (College Station, TX: StataCorp LP). The study received ethics approval from the Rwanda National Ethics Committee (Kigali, Rwanda) and the Partners Healthcare Research Committee (Boston, USA).
Results
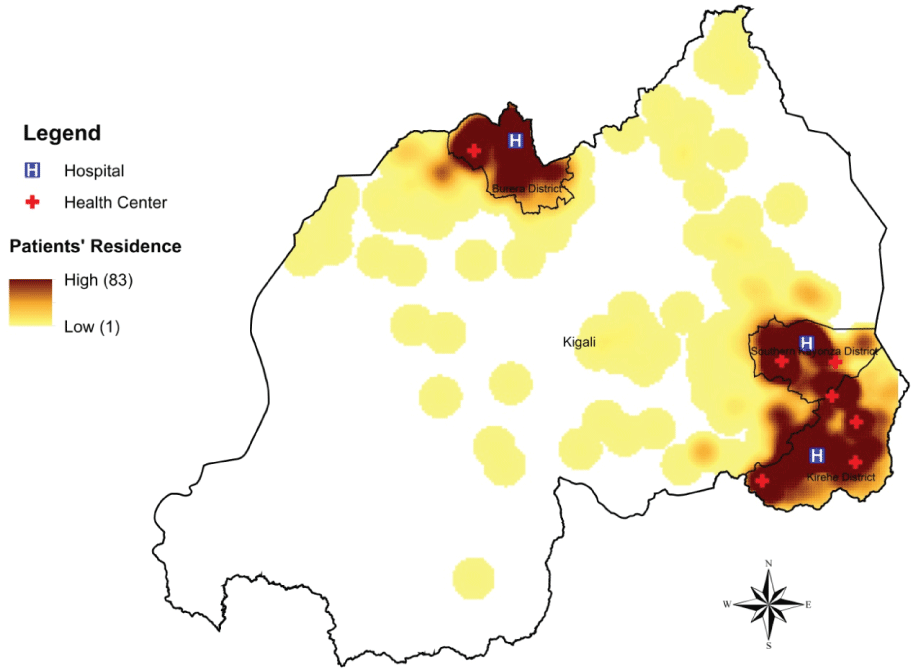
Of the 544 patients enrolled for diabetes care, 305 (56.1%) were female (Table 1). Twenty-two (4.0%) patients were younger than 18 years at enrollment, while 68 (12.5%), 350 (64.3%) and 104 (19.1%) were aged between 18-30 years, 31-60 years and over 60 years, respectively. Most patients (n = 272, 72.5% out of the 375 adults with documented marital status) were married and 163 (50.3% of the 324 adults with number of children documented) had six or more children. The majority of patients (n = 274, 72.3%, of the 379 adults with documented occupation status) were subsistence farmers. Nearly all patients (n = 536, 98.5%) resided in either Northern or Eastern Province and most were residents of the three PIH-supported districts (Figure 1).
![]()
Table 1: Baseline sociodemographic characteristics of diabetic patients managed at PIH-supported MOH NCD programs.
View Table 1
For the 287 with type of diabetes documented, the majority (n = 248, 86.4%) had Type 2 diabetes (Table 2). A third (n = 133, 35.8%, of the 371 with documented baseline BMI) had baseline BMI of 25 or more and 52 (14.0%) had BMI less than 18. Only 22 (5.3% of the 418 with documented status) of diabetes patients were current smokers and 56 (13.9% of the 402 with documentation) currently consumed alcohol of any quantity. Only 53 (9.7%) patients had a documented chronic disease comorbidity: 28 with hypertension, 19 with HIV, three with asthma, two with heart failure and one with heart failure and asthma.
![]()
Table 2: Baseline clinical characteristics of diabetic patients managed at PIH-supported MOH NCD programs.
View Table 2
Of the 544 diabetes patients ever enrolled, 30 patients did not return following initial visit. For the 478 patients with at least 12 months between enrollment and the end of the study period, 55 (11.5%) patients were LTFU and eight (1.7%) died within the first 12 months (Table 3). The majority (n = 415, 86.8%) were alive and in-care at 12 months. For the 394 patients with at least 24 months between enrollment and the end of the study period, 66 (16.8%) patients were LTFU and 12 (3.0%) died within the first 24 months and 316 (80.2%) of patients were alive and in-care at 24 months.
![]()
Table 3: Outcomes among diabetic patients followed at baseline and 6, 12, 18 and 24 months.
View Table 3
For the 70 patients with HbA1c test results documented at baseline (within three months of first visit), median baseline HbA1c was 10.3% DCCT (inter quartile range (IQR: 8.3, 11.9). At four to six months of follow-up, median HbA1c was 8.5% (IQR: 6.0, 11.0, N = 29). At seven to 12 months of follow-up, median HbA1c was 8.2% (IQR: 6.3, 10.1, n = 64). At 13 to 18 months follow-up, median HbA1c was 8.4% (IQR: 6.7, 9.4, n = 70). At 19 to 24 months follow-up, median HbA1c was 8.6% (IQR: 6.5, 10.2, n = 71). Among all patients who had HbA1c results documented at both baseline and seven to 12 month time period (n = 16), median HbA1c was 10.9 (IQR: 7.8, 12.6) and 7.2 (IQR: 6.3, 11), respectively (p = 0.04). Among patients with baseline HbA1c > 10, nine had HbA1c documented at baseline and at 7 to 12 months follow up interval; median HbA1c was 12.2 (IQR: 11.6, 13) and 6.4 (IQR: 5.9, 11), respectively (p = 0.01).
Discussion
Our findings indicate high 24-month retention rates for diabetes patients in a predominantly rural, poor population residing in a low-income country. Further, for patients who are enrolled and retained in the program, reasonable glycemic control has been achieved and maintained over a long time. The baseline HbA1c values seen among our patients are higher than those in patients in developed countries [29,30], and comparable to those in the region [6,10,23].
Few studies in the region report survival, retention or LTFU rates among diabetic patients. A descriptive study of diabetic and hypertensive patients from Cameroon reported a LTFU rate (defined as no visit for three or more months) of 70.6% with median follow up of 17.6 months [12]. Results from a cluster randomized trial of hypertensive and diabetic patients in rural Cameroon reported 29% retention at 12 months in the control arm, 60% retention in patients that received medication subsidies and 65% retention in patients that received missed visit reminder letters [31]. Our patient outcomes were considerably better than these two studies with 16.8% LTFU at twenty-four months. However, there remains room for improvement in patient retention. It is possible that relocation of patients to other facilities outside of the PIH catchment area, which was not routinely captured, inflated our LTFU rates and we recommend that this be better documented in the future. Further, the NCD program recently started active tracing of LTFU patients and determination of death using home visits by community health workers and contacting next of kin. This will help the program better understand the needs of patients LTFU for developing appropriate interventions.
While type of diabetes was missing for nearly half of our patients, 86% of those with documented type were type 2. Type 2 diabetes is estimated to comprise well over 90% of cases in Africa [32,33], which is comparable to 85% - 95% of diabetes cases in high income countries (HICs) [2]. Differences in characteristics between type 1 diabetes in low income and high income settings, such as later age of onset and distinct risk factors, may exist and have been suggested in studies in the region [33-37]. We did not have sufficient type 1 diabetes patient numbers to explore characteristics within this group. Our setting also does not allow for islet autoantibody and C-peptide testing that can evaluate for diagnosis of type 1 diabetes in patients where diagnosis type may be less clear (adolescents or young adults, obese) [38]. Starting in 2015, systematic evaluation with blood glucose testing of children and adolescents with symptoms suggestive of diabetes will be conducted at all health centers in Southern Kayonza District and characterization with C-peptide and islet autoantibody testing will be conducted for a subset of patients.
The World Health Organization (WHO) attributes NCD burden to four main lifestyle risk factors: unhealthy diet, lack of physical activity, tobacco use, and harmful alcohol use [1,39]. Our patient population, predominantly rural based, does not have high proportions of the typical lifestyle risk factors of smoking, harmful alcohol use, sedentary lifestyle or obesity. The low proportion of overweight and obese individuals (36%) is consistent with findings of rural communities in other settings [40] but contrasts with 68% prevalence observed in the United States' general population in 2007-8 [41] and 94% prevalence observed among type 2 diabetics followed at secondary care clinics in the United Kingdom [42]. That nearly three quarters of patients with documented occupation are subsistence farmers also is an indication of relatively active lifestyle. Our findings are consistent with studies that have found that traditional risk factors for diabetes such as higher income, higher education level and urban living play a lesser role in settings of rural poverty [3,33,43]. Some headway has been made with population-based risk factor studies and national surveillance (World Health Organization STEP-wise Approach to Surveillance, Demographic and Health Survey), however, more data are needed to properly assess true burden of diabetes, assess risk factors and outcomes in LMICs [3,24].
Limitations
Our study has some notable limitations. The retrospective design of the study and reliance on routinely collected electronic health data limited data availability. A likely cause of missing data related to HbA1c is the evolving system of health information collection. Early in the program clinical forms for diabetes did not have a specific field for recording HbA1c, so clinicians either left this undocumented or annotated the paper chart. Since December 2011, a specific HbA1c field was added to both paper and electronic forms to preclude this paper and electronic chart inconsistency. However, even with this contribution to missing data, it was evident that many patients did not receive HbA1c testing according to protocol, which specifies that all patients should have at least one HbA1c check per year and more frequently for those whose medications are being actively titrated. Reasons for suboptimal testing include stock-out of test cartridges, clinicians' inability to perform the point-of-care test due to high clinic patient volume or not realizing that patient is due for routine test. In the future, we plan to optimize HbA1c procurement systems by increasing threshold stock volumes that trigger new stock to be ordered, so that there is more lead time before stock outs occur. Furthermore, additional staff has been hired to support the needs of growing patient numbers and the electronic medical records system is being used to generate alerts when patients are due for HbA1c testing.
Given limitations on medication details in the EMR, we were not able to exclude patients who were already on treatment at time of enrollment. Inclusion of baseline HbA1c for patients on treatment could falsely lower the baseline HbA1c. We anticipate that few patients had previous treatment at the time of enrollment (given relative scarcity of other programs providing NCD care) and that this impact is small. We were not able to capture complications such as hypoglycemic episodes, neuropathy, foot ulceration and retinopathy; while these are assessed for clinically they are not routinely recorded. Finally, the LTFU rate may be lower than reported for two reasons. First, the system lacked standardized documentation of patient transfers, so patients who transferred care to a facility outside the catchment area may have been classified as LTFU. Second, pharmacy visits for medication refills were not documented in the system. As such, patients who were not seen by a clinic provider in six months but continued to pick up medications were documented as LTFU. Efforts are underway to systematize phone and community health worker-supported contact with patients who miss visits and to facilitate active death notification. Our findings may be hypothesis-generating; further studies are needed to describe longer term outcomes, to identify effective interventions to reduce LTFU, and to explore context specific risk factors of disease.
Conclusion
In conclusion, our study demonstrates that relatively high retention and low rates of death in patients enrolled in the diabetes program can be achieved, and that for patients who remain enrolled diabetes can be effectively managed with reasonable and sustained glycemic control in a rural resource-limited and multi-center setting. We believe that enabling factors include task-shifting to nurses, embedding services within the rural setting so that they are closer to the homes of patients in need, decentralizing services to more facilities with the support of structured training and strong mentorship, employing point-of-care testing to minimize wait times and frequency of visits, and addressing socioeconomic needs for patients who are particularly vulnerable. However, contributions of these program features, both combined and individually, should be studied more in the future. Further, we found that our diabetes patients in rural Rwanda had low levels of lifestyle risk factors and comorbidities compared with patients in the United States and Europe. These findings may have implications for context-specific prevention and control strategies for diabetes in similar settings and should be further studied with population-level NCD risk factors studies.
Author Contributions
NT conducted literature review, contributed to conception of manuscript, supported data extraction and cleaning, performed data analysis and interpretation, and led writing and critical review of the manuscript. HH conducted literature review, performed data extraction and cleaning and contributed to writing and critical review of manuscript. SA performed data analysis and interpretation, and contributed to writing and critical review of manuscript. CB and GB contributed to the conception, data interpretation, critical review and revision of the manuscript. BLHG supported data interpretation and performed critical review and editing of manuscript. SD, EH, FM, PFU, CM, GN, DN and ER contributed to organization and critical review of manuscript.
Acknowledgments (including sources of funding)
The authors would like to thank the following without whose dedicated work, institutional support, leadership and collaboration this work would not be possible: the Non-Communicable Diseases program and Health Information Systems teams at Rwinkwavu, Kirehe and Butaro hospitals, Brigham and Women's Hospital Division of Global Health Equity, Harvard Medical School Department of Global Health and Social Medicine Research Core, Non-Communicable Diseases Writing Group, Rwanda Ministry of Health leadership, National University of Rwanda, Fulgence Nkikabahizi, Jean Nepomuscene Uwilingiyemungu, Tharcisse Mpunga, Eric Ngabireyimana, Gene Kwan, Egide Mpanumusingo, Rose Nyirahabimana, Cyprien Gahamanyi, Paul Park, Fabien Munyaneza, Ryan Borg, Evariste Ntaganda, Marie Aimee Muhimpundu, and Jean De Dieu Ngirabega. We are also grateful to the Medtronic Foundation, the Helmsley Charitable Trust, Brigham and Women's Hospital, Partners In Health, Rwanda Ministry of Health and Rwanda Biomedical Center for funding and other supports for patient care that have facilitated delivery of these patient care services. SA received support from the Rose Traveling Fellowship. BLHG received research support from the Department of Global Health and Social Medicine Research Core at Harvard Medical School.
Conflicts of Interest
The authors (NT, HH, SA, EH, FM, BLHG, SD, PFU, CM, GN, DN, ER, GB, CB) declared no conflict of interest and none have been paid to write this article by a pharmaceutical company or other agency. This study was not funded by any outside organization. The funders for PIH-supported NCD program had no role in study design, data collection, data analysis, data interpretation or writing of this manuscript. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication. The authors confirm that this study has not been submitted to any other medical journal, and has not been previously published in any medical journal.
References
-
(2011) World Health Organization: Global status report on non-communicable diseases 2010.
-
(2013) International Diabetes Federation: IDF Diabetes Atlas (6th edn).
-
Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, et al. (2011) Non-communicable diseases in sub-Saharan Africa: What we know now. Int J Epidemiol 40: 885-901.
-
Bukhman G, Bavuma C, Gishoma C, Gupta N, Kwan GF, et al. (2015) Endemic diabetes in the world's poorest people. Lancet Diabetes Endocrinol 3: 402-403.
-
Tesfaye S, Gill G (2011) Chronic diabetic complications in Africa. African Journal of Diabetes Medicine 19: 36-40.
-
Gill GV, Price C, Shandu D, Dedicoat M, Wilkinson D (2008) An effective system of nurse-led diabetes care in rural Africa. Diabet Med 25: 606-611.
-
Kengne AP, Amoah AGB, Mbanya JC (2005) Cardiovascular complications of diabetes mellitus in sub-Saharan Africa. Circulation 112: 3592-3601.
-
Alemu S, Watkins VJ, Dodds W, Turowska JB, Watkins PJ (1998) Access to diabetes treatment in Northern Ethiopia. Diabet Med 15: 791-794.
-
Price C, Shandu D, Dedicoat M, Wilkinson D, Gill GV (2011) Long-term glycaemic outcome of structured nurse-led diabetes care in rural Africa. QJM 104: 571–574.
-
Lekoubou A, Awah P, Fezeu L, Sobngwi E, Kengne AP (2010) Hypertension, diabetes mellitus and task shifting in their management in sub-Saharan Africa. Environ. Res. Public Health 7: 353-363.
-
Coleman R, Gill G, Wilkinson D (1998) Noncommunicable disease management in resource-poor settings: A primary care model from rural South Africa. Bull World Health Organ 76: 633-640.
-
Labhardt ND, Balo JR, Ndam M, Grimm JJ, Manga E (2010) Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: A programme assessment at two years. BMC Health Serv Res 10: 339.
-
Kengne AP, Fezeu L, Sobngwi E, Awah PK, Aspray TJ, et al. (2009) Type 2 diabetes management in nurse-led primary healthcare settings in urban and rural Cameroon. Prim Care Diabetes 3: 181-188.
-
Windus DW, Ladenson JH, Merrins CK, Seyoum M, Windus D, et al. (2007) Impact of a multidisciplinary intervention for diabetes in Eritrea. Clin Chem 53: 1954-1959.
-
Rudasingwa GJ, Amendezo E, Twagirumukiza M (2012) Clinical patterns and complications of African diabetic patients: Preliminary data from Kigali University Teaching Hospital, Rwanda. African Journal of Diabetes Medicine 20: 39-42.
-
Acheampong JW, Boeteng KA, Egham BA, Story P, Parry EHO, et al. (2000) The impact of diabetes nurses in the Komfo Anokye Teaching Hospital, Ghana. Diabet Int 10: 81-93.
-
Bukhman G, Kidder A (2011) The Partners In Health guide to chronic care for endemic non-communicable diseases. Rwanda edition. Cardiac, renal, diabetes, pulmonary, and palliative care. Boston, United States.
-
Tapela NM, Bukhman G, Ngoga G, Kwan GF, Mutabazi F, et al. (2015) Treatment of non-communicable disease in rural resource-constrained settings: a comprehensive, integrated, nurse-led care model at public facilities in Rwanda. The Lancet Global Health 3: S36.
-
Worldbank open data-Rwanda Country prorile
-
Oxford Poverty and Human Development Initiative (2015). "Rwanda Country Briefing", Multidimensional Poverty Index Data Bank. January 2015: Rwanda. OPHI, University of Oxford, 2015.
-
Binagwaho A, Farmer PE, Nsanzimana S, Karema C, Gasana M, et al. (2014) Rwanda 20 years on: Investing in life. Lancet 384: 371-375.
-
Ministry of Health Rwanda, Health Sector Annual Report, July 2013 - June 2014.
-
Marshall SL, Edidin DV, Arena VC, Becker DJ, Bunker CH, et al. (2014) Glucose control in Rwandan youth with type 1 diabetes following establishment of systematic, HbA1c based, care and education. Diabetes Res Clin Pract 107: 113-122.
-
(2013) International Diabetes Federation: Rwanda - Extract of the Global Diabetes Scorecard Tracking Progress for Action.
-
Mukeshimana MM, Nkosi ZZ (2014) Communities' knowledge and perceptions of type two diabetes mellitus in Rwanda: A questionnaire survey. J Clin Nurs 23: 541-549.
-
Kwan GF, Bukhman AK, Miller AC, Ngoga G, Mucumbitsi J, et al. (2013) Simplified echocardiographic strategy for heart failure diagnosis and management at district hospital level for sub-Saharan Africa. JACC Heart Failure 1: 230-236.
-
Anatole M, Magge H, Redditt V, Karamaga A, Niyonzima S, et al. (2013) Nurse mentorship to improve the quality of health care delivery in rural Rwanda. Nurs Outlook 61: 137-144.
-
Magge H, Anatole M, Cyamatare FR, Mezzacappa C, Nkikabahizi F, et al. (2014) Mentoring and quality improvement strengthen integrated management of childhood illness implementation in rural Rwanda. Arch Dis Child 100: 565-570.
-
Looker HC, Nyangoma SO, Cromie D, Olson J, Leese GP, et al. (2012) Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia 55: 2335-2342.
-
Koro C, Bowlin S, Bourgeois N, Fedder D (2004) Glycemic Control from 1988 to 2000 among US adults diagnosed with type 2 diabetes. Diabetes Care 27: 17-20.
-
Labhardt ND, Balo JR, Ndam M, Manga E, Stoll B (2011) Improved retention rates with low-cost interventions in hypertension and diabetes management in a rural African environment of nurse-led care: A cluster-randomised trial. Trop Med Int Health 16: 1276-1284.
-
Hall V, Thomsen RW, Henriksen O, Lohse N (2011) Diabetes in Sub Saharan Africa 1999-2011: Epidemiology and public health implications. A systematic review. BMC Public Health 11: 564.
-
Mbanya JCN, Motala A, Sobngwi E, Assah FK, Enoru ST (2010) Diabetes in sub-Saharan Africa. Lancet 375: 2254-2266.
-
Swai ABM, Lutale J, McLarty DG (1990) Diabetes in tropical Africa: A prospective study 1981-7. I. Characteristics of newly presenting patients in Dar es Salaam, Tanzania, 1981-7. BMJ 300: 1103-1106.
-
Omar MAK, Asmal AC (1984) Patterns of diabetes mellitus in young African and Indians in Natal. Trop Geogr Med 36: 133-138.
-
Kalk WJ, Huddle KRL, Raal FJ (1993) The age of onset and sex distribution of insulin-dependent diabetes mellitus in Africans in South Africa. Postgrad Med J 69: 552-556.
-
Fekadu S, Yigzaw M, Alemu S, Dessie A, Fieldhouse H, et al. (2010) Insulin-requiring diabetes in Ethiopia: Associations with poverty, early undernutrition and anthropometric disproportion. Eur J Clinl Nutr 64: 1192-1198.
-
Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, et al. (2005) Care of children and adolescents with Type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care 28: 186-212.
-
(2010) Package of Essential Non-communicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. World Health Organization, Geneva.
-
Neuman M, Finlay JE, Davey Smith G, Subramanian S (2011) The poor stay thinner: Stable socioeconomic gradients in BMI among women in lower- and middle-income countries. Am J Clin Nutr 94: 1348-1357.
-
Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303: 235-241.
-
Daousi C, Casson IF, Gill GV, MacFarlane I, Wilding JPH (2006) Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J 82: 280-284.
-
Sobngwi E, Mbanya JC, Unwin NC, Porcher R, Kengne AP, et al. (2004) Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int J Epi 33: 769-776.