International Journal of Diabetes and Clinical Research
Glucose Tolerance is Improved Following Surgery for Silent Somatotroph Adenoma
Aika Miya1, Akinobu Nakamura1*, Hideaki Miyoshi1, Hiraku Kameda1, Hiroshi Nomoto1, So Nagai1, Yuko Omori2, Kanako C. Hatanaka2, Hiroyuki Kobayashi3, Chikara Shimizu4, and Tatsuya Atsumi1
1Division of Rheumatology, Endocrinology and Nephrology, Hokkaido University Hospital North 15, West 7, Kita-ku, Sapporo, Hokkaido, 060 - 8638, Japan
2Department of Surgical Pathology, Hokkaido University Hospital North 15, West 7, Kita-ku, Sapporo, Hokkaido, 060-8638, Japan
3Department of Neurosurgery, Hokkaido University Hospital North 15, West 7, Kita-ku, Sapporo, Hokkaido, 060-8638, Japan
4Division of Laboratory and Transfusion Medicine, Hokkaido University Hospital North 15, West 7, Kita-ku, Sapporo, Hokkaido, 060-8638, Japan
*Corresponding author:
Akinobu Nakamura, MD, PhD, Division of Rheumatology, Endocrinology and Nephrology, Hokkaido University Graduate School of Medicine North 15, West 7, Kita-ku, Sapporo, Hokkaido, 060-8638, Japan, Tel: +81-11-706-5915, Fax: +81-11-706-7710 E-mail:
akinbo@tim.hi-ho.ne.jp
Int J Diabetes Clin Res, IJDCR-2-047, (Volume 2, Issue 6), Case Report; ISSN: 2377-3634
Received: August 02, 2015 | Accepted: November 02, 2015 | Published: November 05, 2015
Citation: Miya A, Nakamura A, Miyoshi H, Kameda H, Nomoto H, et al. (2015) Glucose Tolerance is Improved Following Surgery for Silent Somatotroph Adenoma. Int J Diabetes Clin Res 2:047. 10.23937/2377-3634/1410047
Copyright: © 2015 Miya A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Although the excessive secretion of GH leads to insulin resistance enhancement, the involvement of a silent somatotroph adenoma in abnormal glucose tolerance has not been elucidated. A 50 - year-old man was admitted with a headache and bitemporal hemianopia caused by a pituitary macroadenoma. He had no physical signs and symptoms of acromegaly nor hypopituitarism, and his base-line serum levels of GH and insulin - like growth factor-1 (IGF-1) were normal. However, a 75 - g oral glucose tolerance test (OGTT) showed unsuppressed GH concentrations as well as plasma glucose levels consistent with diabetes pattern. A transsphenoidal adenomectomy was performed, and we diagnosed the patient as having a silent somatotroph adenoma based on positive GH in the immunohistochemistry. Postoperative OGTT showed GH suppression and a normal pattern of plasma glucose levels after glucose loading. The post-surgery homeostasis model assessment insulin resistance index (HOMA - IR) and the Matsuda Index indicated improved insulin sensitivity in the absence of perioperative body-mass-index change. These observations suggest that reversible abnormal glucose tolerance is associated with a silent somatotroph adenoma in this patient.
Keywords
Silent somatotroph adenoma, glucose tolerance, oral glucose tolerance test, insulin sensitivity.
Introduction
Chronic exposure to excessive GH is associated with abnormal glucose tolerance through an increase in hepatic gluconeogenesis, the reduction of glucose uptake in the muscle, and the activation of intracellular lipolysis and lipid oxidation [1]. Therefore, glucose intolerance is a well - known comorbidity in patients with acromegaly [2]. It has been reported that insulin resistance improves after the suppression of GH activity through successful surgical treatment [3-5].
Silent somatotroph adenoma is recognized as acromegaly in the absence of any clinical signs of classical acromegaly (growth of hands, feet, head or jaw in adulthood, frontal bossing, prognathism, large tongue, and wide hands and feet) and not more than two associated signs as those seen in the general population but more commonly in acromegaly (carpal tunnel syndrome, obstructive sleep apnea, type 2 diabetes, hypertension, and colonic polyps) nor elevated serum concentrations of insulin - like growth factor-1 (IGF-1), but positive GH in the immunohistochemistry [6,7]. The frequency of silent somatrotroph adenoma is regarded as sporadic, accounting for 4.2% of somatotroph adenomas [6]. However, the involvement of a silent somatotroph adenoma in abnormal glucose tolerance has not been elucidated.
We report a patient with silent somatotroph adenoma, successfully treated by pituitary surgery. His insulin sensitivity increased following surgery without any significant change of his body-mass-index (BMI). The observation has rendered us to discuss the association between reversible glucose tolerance disorder and silent somatotroph adenoma.
Case Presentation
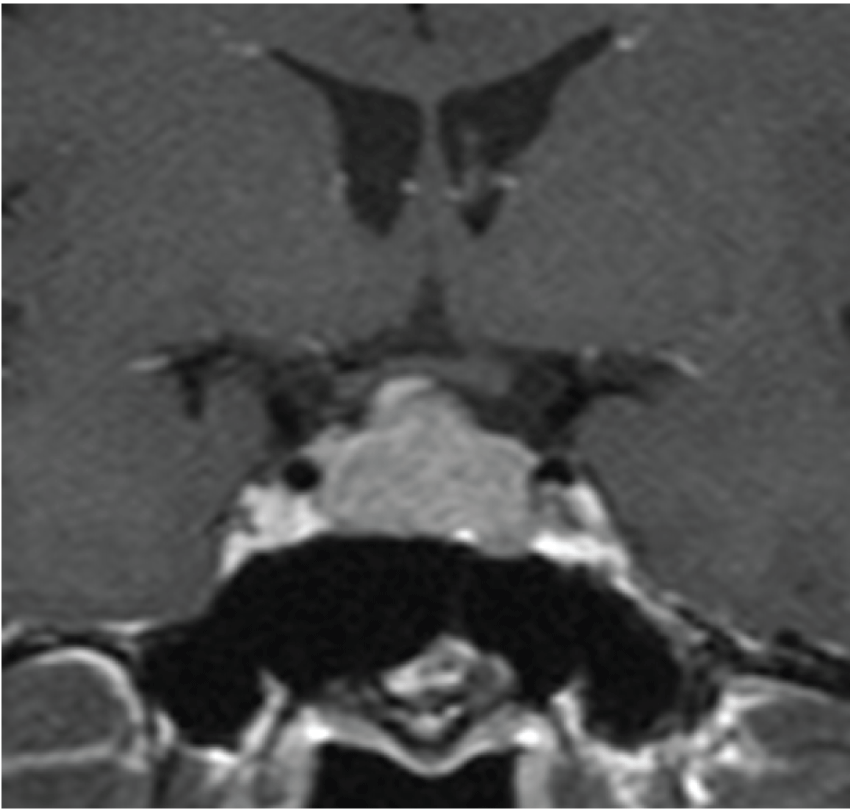
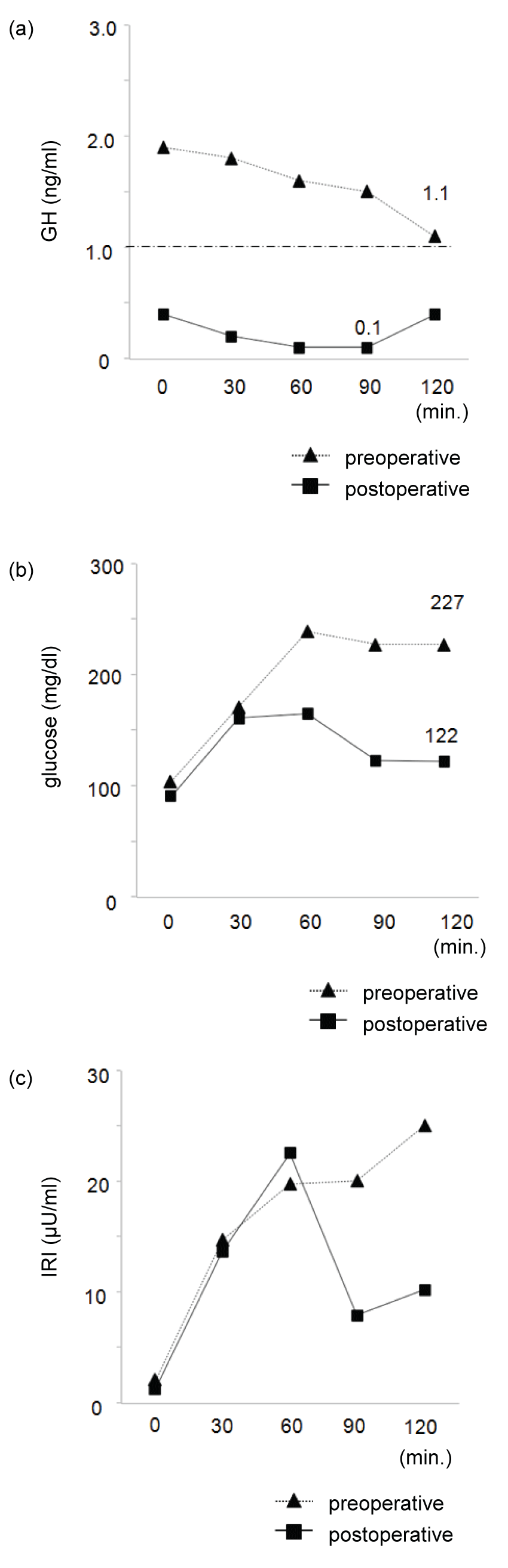
A 50 - year - old man in previous good health who had suffered from a headache for one week was admitted. Magnetic resonance imaging (MRI) revealed a single enhanced pituitary mass (measuring 16*15 mm) extending to the suprasellar area with compression of the optic chiasm (Figure 1). On admission, the patient was 175.8 cm tall and weighed 67.1 kg, with BMI of 21.7 kg/m2. His visual fields were characterized by bitemporal hemianopia. But he had no physical signs or symptoms of acromegaly, including somatic enlargement, excessive sweating, jaw overgrowth, joint pains, carpal tunnel syndrome, or sleep apnea syndrome. Also, fatigue and weakness suggesting hypopituitarism were not described. He had not taken any medications, such as those for diabetes and hypertension. And he had no history of cardiovascular disease, polyps, or dental malocclusion. He was a non - smoker who did not drink alcohol regularly, and he had no allergies. His father had been treated for type 2 diabetes and hypertension. The family history was negative for growth disorders or other forms of endocrinopathy. His GH level measured in the morning fasting was 3.30 ng/mL slightly elevated above normal range, although his serum IGF-1 level was 201 ng/mL (+0.8 SD). Also, his hemoglobin A1c (HbA1c) level was 5.8%. Since it has been reported that a 75 - g oral glucose tolerance test (OGTT) is desirable if HbA1c is 5.6 - 5.9% [8], we performed OGTT in our case as assessment of glucose tolerance and the high GH level in the morning fasting. As shown in figure 2a, his GH levels did not suppress during OGTT. The OGTT also revealed a diabetic pattern at 120 minutes (Figure 2b and Figure 2c). His prolactin level was 11.4 ng/mL. Other routine laboratory tests, including tests for liver function, LH, FSH and testosterone, thyroid function, serum cortisol and ACTH were all normal shown in table 1, although it was suggested that hypopituitarism was likely because of tumor compression on normal pituitary tissue.
.
Figure 1: Preoperative coronal MRI showing an enhanced pituitary tumor extending to the suprasellar area with compression of the optic chiasm.
View Figure 1
![]()
Table 1: Laboratory data on admission
View Table 1
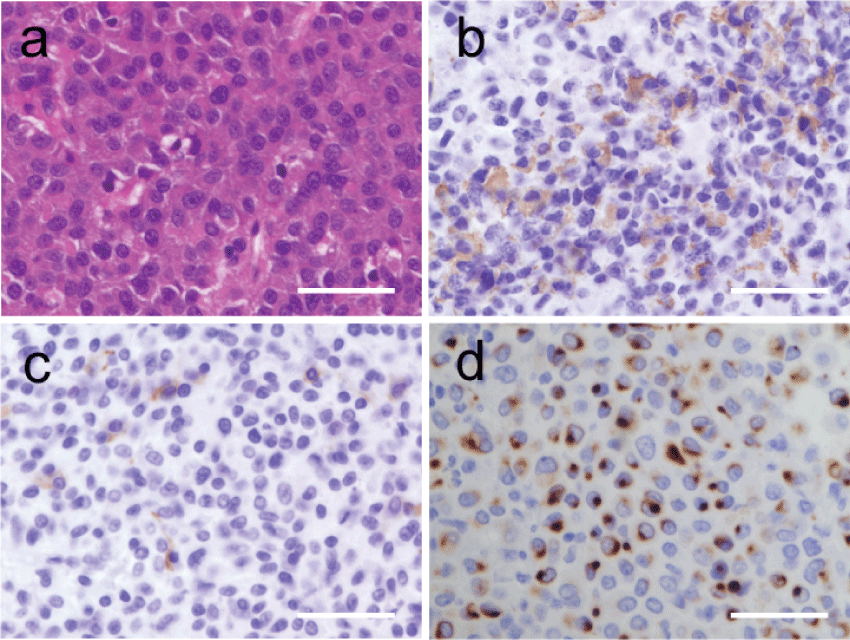
To alleviate the symptoms of headache and bitemporal hemianopia, a transsphenoidal adenomectomy was performed. He was covered with hydrocortisone succinate 100 mg on the day of surgery for potential hypopituitarism. Hydrocortisone succinate was slowly tapered within 7 days of surgery. He has taken hydrocortisone 15 mg a day in divided doses since postoperative day 8. The pathological findings of the surgically obtained tumor showed an adenoma composed of chromophobic cells when stained using hematoxylin and eosin (H & E) (Figure 3a). Immunohistochemical staining revealed GH positivity in the majority of the tumor cells, whereas prolactin immunoreactivity was localized (Figure 3b and Figure 3c). Fibrous bodies, representing pale acidophilic, spherical inclusions in the cytoplasmic area, were strongly reactive for low molecular weight cytokeratins, particularly keratin 8/18 (Figure 3d). In immunohistochemical staining, we have used primary antibodies against GH (1:4000, poly; Dako), PRL (1:1000, SPM108; Thermo), and CK 8/18 (CAM 5.2, 1:200, 5D3; Novocastra). These findings corresponded to a sparsely granulated somatotroph adenoma [9]. Based on positive GH in the immunohistochemistry despite the absence of any clinical signs of classical acromegaly except only abnormal glucose tolerance nor elevated serum concentrations of IGF-1, we diagnosed the pituitary macroadenoma as a silent somatotroph adenoma [6]. The postoperative course was uneventful. His visual field returned to normal, and his headache disappeared, although hypopituitarism persisted shown in table 2. He has taken adequate hormonal replacement therapy of hydrocortisone 15 mg a day in divided doses and levothyroxine 50 μg a day. Hormone levels during replacement therapy on maintenance doses were fT3 2.69 pg/mL, fT4 1.33 ng/dL, the 24 - hour urine free cortisol 35.5 μg/day all within normal range. He had a regular diet and kept quiet without exercise therapy in the perioperative period.
.
Figure 2: Serum GH (a), glucose (b), and insulin (c) levels were measured at 30-minute intervals before and for 120 minutes after a 75-g OGTT.
View Figure 2
![]()
Table 2: Postoperative function testing of GH, LH, FSH, TSH, ACTH, cortisol and PRL after administration of GHRP-2, TRH, LHRH and CRH.
View Table 2
On postoperative day 28, we evaluated the effects of the surgical treatment using an OGTT. His nadir GH levels were suppressed to less than 1.0 ng/mL (Figure 2a). Furthermore, the OGTT showed a normal pattern of plasma glucose levels after glucose loading (Figure 2b and Figure 2c). His IGF-1 level decreased to 100 ng/mL (-1.5 SD). Compared with his preoperative values shown in table 3, the homeostasis model assessment insulin resistance index (HOMA -IR), calculated as HOMA - IR = (fasting plasma insulin (μU/mL) * fasting plasma glucose (mg/dL))/405 [10], decreased from 0.54 to 0.29, and the Matsuda index of insulin sensitivity, calculated from glucose and insulin levels at 0, 30, 60, 90, and 120 minutes (5 time points) after a 75 - g OGTT [11], increased from 11.59 to 22.12. These results indicated enhanced insulin sensitivity. The insulinogenic index (Δinsulin [0 - 30 minute]/Δglucose [0 - 30 minute]), which estimates early-phase insulin secretion based on an OGTT [12], remained relatively low. Despite a lack of change in his perioperative BMI (from 21.7 to 21.8 kg/m2), the pituitary surgery for the silent somatotroph adenoma improved the patient's glucose tolerance by increasing his insulin sensitivity.
.
Figure 3: (a) The pathological findings of the surgically obtained tumor show an adenoma composed of chromophobic cells when stained using hematoxylin and eosin (H&E). (b) Immunohistochemical staining revealed GH in the majority of the adenoma cells. (c) Prolactin immunoreactivity was localized. (d) Fibrous bodies were strongly reactive for low molecular weight cytokeratins, particularly keratin 8/18. Scale bar, 50 μm. Original magnification, ×400.
View Figure 3
![]()
Table 3: Perioperative clinical parameters of glucose tolerance
View Table 3
Discussion
This is the first report to show that glucose tolerance was improved by the surgical removal of a silent somatotroph adenoma. GH and IGF-1 have opposing effects on insulin sensitivity. Chronic excessive GH secretion causes insulin resistance, while IGF-1 administration increases insulin sensitivity [13]. Excessive amounts of GH worsen insulin resistance in the liver as well as in peripheral tissues, causing insulin insensitivity and hyperinsulinemia. Yakar et al. [14] reported that excess GH secretion in liver IGF-1-deficient (LID) mice led to severe insulin resistance. In contrast, blocking the GH action in LID mice crossed with GH antagonist transgenic mice led to the normalization of insulin sensitivity. In humans, patients with GH receptor deficiency exhibited no cases of diabetes, instead causing enhanced insulin sensitivity with a lower HOMA-IR [15]. Meanwhile in a recent clinical study, IGF-1 levels, as a parameter reflecting overall GH secretion, were significantly correlated with the HOMA - IR in patients with acromegaly [5]. IGF-1 was an independent predictor of abnormal glucose tolerance in patients with acromegaly. The IGF-1 levels at diagnosis were higher in patients with untreated acromegaly accompanied by impaired glucose tolerance and diabetes than in those with normal glucose tolerance, although no difference in the post - OGTT GH levels at the time was seen between the groups [16].
In our case, despite normal levels of circulating IGF-1 and no changes in the perioperative BMI, insulin sensitivity was improved after the surgical removal of the silent somatotroph adenoma. On one hand, as in the mouse models described above, excessive GH would play a critical role in abnormal glucose tolerance. On the other hand, the IGF-1 level is known to vary widely according to the physiological condition, to nutritional status, and even to ethnicity [17]. The basic IGF-1 level in the present patient might have been lower than the lowest level of "normal range", and his IGF-1 level might have been elevated up to normal due to the GH secretion from the silent somatotroph adenoma. Therefore, his "relatively high" IGF-1 level might have been related to glucose tolerance as well.
The risk factors for progression to diabetes in patients with acromegaly include higher GH levels and a family history of diabetes [1]. If the compensatory hyperfunction of pancreatic β-cells does not counterbalance the reduced insulin sensitivity, abnormal glucose tolerance is likely to become clinically evident [18]. Kinoshita et al. [19] reported that abnormal glucose tolerance fails to normalize after acromegaly has been cured once the function of the pancreatic β-cells has been impaired. In the present case, the insulinogenic index suggested that the impairment in insulin secretion persisted after surgery. The patient had a family history of type 2 diabetes, and his impaired insulin secretion appeared to be an inherent characteristic frequently seen among Asian populations. This case suggests that a slight excess of GH secretion by a silent somatotroph adenoma can cause insulin resistance through the failure of a compensatory increase in β-cell function. Despite the impairment in insulin secretion, his glucose tolerance ultimately improved due to increased insulin sensitivity following surgery for the pituitary macroadenoma.
As part of the management of glucose intolerance in patients with silent somatotroph adenomas, we speculate that excess of GH secretion by a silent somatotroph adenoma should be suppressed by pituitary surgery better than medication. The reasons for this are long acting somatostatin analogues (SSA) used medical treatment of acromegaly reduce β-cell function and elevate blood glucose [16]. While, pegvisomant (PEG) prevents GH signal transduction and increases hepatic and peripheral insulin sensitivity. But lack of GH signaling by GH receptor gene-deficiency induces diminished β-cell mass, insulin gene expression and insulin secretion [20]. In the case that had impaired insulin secretion like ours, there is clinical concern about potential negative effects on glucose metabolism of long SSA and PEG. Additional clinical trials are needed to elucidate what kind of treatment is performed better, operation, medication, or observation.
Although hypopituitarism affected insulin sensitivity generally, hormone levels during replacement therapy on maintenance doses in our case remained unchanged compared with his preoperative values. Furthermore some reports have shown that hypopituitarism such as hypogonadotropic hypogonadism leads to insulin resistance enhancement [21]. But the improvement in glucose tolerance following hypopituitarism has not been elucidated.
The present case report has some limitations. The first one is the definition of silent somatotroph adenoma. Cases of silent somatotroph adenoma have rarely been reported, and the diagnostic criteria remain controversial [22]. Some reports have defined silent somatotroph adenoma as a pituitary adenoma with the immunohistochemical features of somatotroph cells but with neither clinical nor laboratory evidence of hormone excess [23,24]. Whether a pituitary adenoma that is associated with either slightly elevated or normal GH and IGF-1 level can be diagnosed as a silent somatotroph adenoma is debatable. The second limitation is the absence of any measurements of body composition in our case. A reduction in the fat mass leads to an improvement in glucose tolerance. Our case was not obese, had a regular diet, kept quiet in the perioperative period and his perioperative BMI remained unchanged. But the patient's body composition might have altered, such as a reduction in fat mass and an increase in lean body mass during the course of hospitalization. Another limitation of our case is related to one single case. Therefore, we should evaluate glucose tolerance and insulin sensitivity in more patients of silent somatotroph adenoma.
Meanwhile, this pituitary tumor was diagnosed as a GH - secreting tumor with the failure of GH suppression during an OGTT. Some asymptomatic pituitary adenomas, which are diagnosed as simple non - functional tumors because of normal base - line serum levels of GH and IGF-1, might be GH-secreting tumors. Such cases should be diagnosed as having silent somatotroph adenomas and have reversible abnormal glucose tolerances. We should evaluate the GH secretory property of asymptomatic non-functional pituitary adenomas using an OGTT.
In conclusion, our patient's tumor was an unprecedented case of a silent somatotroph adenoma in which the glucose tolerance improved following pituitary surgery. These observations suggest that abnormal glucose tolerance caused by a silent somatotroph adenoma can be curable.
Acknowledgment
This work was supported in part by Health Labor Sciences Research Grant from The Ministry of Education, Culture, Sports, Science, and Technology, Japan (CS).
Consent
Written informed consent was obtained by the patient for the publication of this case report and any accompanying images.
Disclosure
The authors declare that they have no conflict of interest.
References
-
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25: 102-152.
-
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, et al. (2011) American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Acromegaly. Endocr Pract 17: 1-44.
-
Nabarro JD (1987) Acromegaly. Clin Endocrinol 26: 481-512.
-
Sonksen PH, Greenwood FC, Ellis JP, Lowy C, Rutherford A, et al. (1967) Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. J Clin Endocrinol Metab 27: 1418-1430.
-
Mori K, Iwasaki Y, Kawasaki-Ogita Y, Honjo S, Hamamoto Y, et al. (2013) Improvement of Insulin Resistance Following Transsphenoidal Surgery in Patients with Acromegaly: Correlation with Serum IGF-1 Levels. J Endocrinol Invest 36: 853-859.
-
Wade AN, Baccon J, Grady MS, Judy KD, O'Rourke DM, et al. (2011) Clinically silent somatotroph adenomas are common. Eur J Endocrinol 165: 39-44.
-
Cooper O, Melmed S (2012) Subclinical hyperfunctioning pituitary adenomas: the silent tumors. Best Pract Res Clin Endocrinol Metab 26: 447-460.
-
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, et al. (2010) Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 1: 212-228.
-
Kontogeorgos G (2005) Classification and pathology of pituitary tumors. Endocrine 28: 27-35.
-
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
-
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462-1470.
-
Onishi Y, Hayashi T, Sato KK, Ogihara T, Kuzuya N, et al. (2010) Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J Diabetes Investig 1: 191-195.
-
Holt RI, Simpson HL, Sonksen PH (2003) The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 20: 3-15.
-
Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, et al. (2004) Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest 113: 96-105.
-
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, et al. (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3: 70.
-
Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, et al. (2014) Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary 17: 81-89.
-
Cruickshank JK, Heald AH, Anderson S, Cade JE, Sampayo J, et al. (2001) Epidemiology of the insulin-like growth factor system in three ethnic groups. Am J Epidemiol 154: 504-513.
-
Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, et al. (2000) Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol (Oxf) 52: 549-555.
-
Kinoshita Y, Fujii H, Takeshita A, Taguchi M, Miyakawa M, et al. (2011) Impaired glucose metabolism in Japanese patients with acromegaly is restored after successful pituitary surgery if pancreatic beta-cell function is preserved. Eur J Endocrinol 164: 467-473.
-
Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, et al. (2004) Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab 287: E405-413.
-
Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. (2007) Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 92: 4254-4259.
-
Mohammed S, Syro L, Abad V, Salehi F, Horvath E, et al. (2009) Silent somatotroph adenoma of the pituitary in an adolescent. Can J Neurol Sci 36: 123-125.
-
Yamada S, Sano T, Stefaneanu L, Kovacs K, Aiba T, et al. (1993) Endocrine and morphological study of a clinically silent somatotroph adenoma of the human pituitary. J Clin Endocrinol Metab 76: 352-356.
-
Kovacs K, Lloyd R, Horvath E, Asa SL, Stefaneanu L, et al. (1989) Silent somatotroph adenomas of the human pituitary. A morphologic study of three cases including immunocytochemistry, electron microscopy, in vitro examination, and in situ hybridization. Am J Pathol 134: 345-353





