International Journal of Diabetes and Clinical Research
Diabetes - A Systemic Risk Factor for the Development of Chronic Periodontitis in Bulgarian Patients
Nina M. Musurlieva* and Mariya S. Bratoycheva
Deparment of Social Medicine and Public Health, Faculty of Public Health, Medical University of Plovdiv, Bulgaria
*Corresponding author:
Nina M. Musurlieva, DDS, PhD, Deparment of Social Medicine and Public Health, Faculty of Public Health, Medical University of Plovdiv, Bul. V. Aprilov 15A, Plovdiv 4000, Bulgaria, Tel: +359 888 312 499, E-mail: nina_mussurlieva@abv.bg
Int J Diabetes Clin Res, IJDCR-2-030, (Volume 2, Issue 3), Research Article; ISSN: 2377-3634
Received: January 26, 2015 | Accepted: April 29, 2015 | Published: May 02, 2015
Citation: Musurlieva NM, Bratoycheva MS (2015) Diabetes - A Systemic Risk Factor for the Development of Chronic Periodontitis in Bulgarian Patients. Int J Diabetes Clin Res. 2:030. 10.23937/2377-3634/1410030
Copyright: © 2015 Musurlieva NM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Diabetes is an important systemic factor for the development of periodontitis. This study examines the association between the diabetes type 2 and development of chronic periodontitis in the sample of Bulgarian patients.
Material: 228 patients with chronic periodontitis were surveyed using a questionnaire specially designed for the study, and a case-control study was carried out.
Results: Applying Multiple Logistic Regression, this study proves that diabetes type 2 is one of the factors with the strongest relative weight in the surveyed sample. A logistic model for assessing the risk of developing chronic periodontitis has been built, in which diabetes has the highest regression coefficient (B=4.195). Of all surveyed with chronic periodontitis, 15% also have diabetes type 2. It is most common in the group aged over 60. Most of the patients have never had their dental calculus removed, which is also a proven risk factor. On the basis of establishing diabetes type 2 as a risk factor in the surveyed sample, we recommend a closer cooperation between dental practitioners, endocrinologists and general practitioners, aimed at prevention and treatment of periodontal diseases.
Keywords
Diabetes type 2, Chronic periodontitis, Risk factors
Introduction
Diabetes is one of the most common metabolic disorders in the world and the prevalence of diabetes in adults has been increasing in the last decades [1]. Diabetes is an important systemic factor for the development of periodontitis. Periodontitis is considered one of the complication of diabetes [2]. In people with diabetes, regardless of the age group, periodontium morbidity is of a much higher prevalence and severity [3-9]. A survey carried out among native Americans in the State of Arizona provides abundant information on the correlation diabetes-periodontal disease [10]. Modern research is based on division into two types of diabetes and the level of metabolic control [11]. There is data evidencing the fact that the severity of the periodontal destruction can be linked to the type of diabetes, the duration of the disease and the level of metabolic control [12-14]. In the attempt to establish a correlation between diabetes and periodontitis, certain factors have to be taken into consideration, factors such as sex, age, dental plaque and calculus, which sometimes act as obscuring factors. There is a significant correlation between the greater incidence and severity of periodontal diseases, on the one hand, and diabetes, age and subgingival calculus, on the other [15]. Numerous epidemiological studies have shown it is a two-way connection. Not only is diabetes a risk factor for the development of periodontitis, but the periodontal tissue infection itself may cause resistance of the said tissues to insulin and thus lead to diabetes [16-22]. The link between the two diseases was proven in accordance with the Bradford Hill criteria [16]. At the World Workshop in 1996, Offenbacher proposed that a new term should be introduced - 'perio-medicine' to reflect this interconnection and emphasize the collaboration between physicians and dental practitioners in the prevention and treatment of these diseases [13].
Aim
On the basis of the above discussion, this study examined association between the diabetes type 2 and development of chronic periodontitis in the sample of Bulgarian patients.
Methods
From November 2010 to February 2011, a study was conducted to assess the quality of life in 228 patients with chronic periodontitis. Participants were randomly selected from outpatients at the Department of Periodontology, Faculty of Dentistry, Medical University of Plovdiv (Bulgaria) and from various dentist surgeries in Plovdiv. All of them had sought treatment in the Department and the dentist surgeries. Prior to the study calibration of all nine periodontistis examined the patients were performed.
Within the frame of the above-mentioned research, a pilot case-control study was conducted, including 80 patients. It aimed to assess the risk of chronic periodontitis in the surveyed patients. The minimum sample size of patients was determined based on power analysis for sample size calculation. Age under 20 years was an exclusion criterion.
The periodontal health status was measured using The Community Periodontal Index (CPI) recommended by the World Health Organization (WHO) as a standard epidemiological examination method for periodontal disease, with dental mirror and WHO periodontal probe. Periodontitis was diagnosed based on the presence of clinically significant inflammation, periodontal osseous pockets and bone destruction. Thus, we selected 60 controls (randomly selected patients without periodontitis who had visited the dentist surgeries for other reasons) and 20 cases out of the 228 patients, included in the primary survey. The cases included patients with chronic periodontitis who were matched to the controls, according to the most important socio-demographic parameters: sex, age, education. The aim was to eliminate the impact of confounding factors. The case/control ratio was 1:3 [23].
A direct, individual interview was performed with the participants from the cases and the control group. From the basic interview questionnaire (developed to assess the quality of life of the 228 patients with chronic periodontitis), only the sections, related to risk factors assessment, were used. The relevant sections were: socio-demographic characteristics of the patients cohort; presence of common risk factors prior to the development of periodontitis: smoking, alcohol abuse, stress, diabetes, vegetarian diet, fresh vegetable and fruit consumption, medication use, presence of hazards in the professional environment, level of oral hygiene, tooth clenching and grinding, regular calculus removal procedures, presence of overlapping and crooked teeth, regularity of dental examinations.
Descriptive statistics was used: analysis of variance, alternative analysis and non-parametric analysis (Fisher's exact test, t-test, Pearson criterion). Multiple logistic regressions were applied to evaluate the association between the variables (BackwardConditional procedure). Statistical significance was assumed at P= 0.05. SPSS ver.13.0 was applied for data processing.
Results and Discussion
Diabetes and periodontitis are both complex chronic diseases. Diabetes is a significant systemic factor for the development of periodontitis. The prevalence estimates of diabetes type 2 in Bulgaria, adults aged 20-79 years, 2013 is 5.3% [24].
Diabetes is most common among 50-year olds - 83%, and the group of that aged 40-49 75.3% [25].
No statistically significant difference was observed in the socio-demographic parameters between cases and controls. Most people in the studied sample were young adults: the average age was 31.33-33.00 years. The number of females was slightly higher than that of males (the former were 56.67% of the patients in the control group and 55% in the cases group). The control group was comprised mostly of patients with secondary education (56.67%). In the cases group, the percentage of patients with higher education was equal to that of patients with secondary education. The patients with mild periodontitis (when attachment loss is 1-2 mm) were 9 (45 ± 11.12%); with moderate (attachment loss 3-4 mm) were 7 (35 ± 10.66%) and with severe form of periodontitis (attachment loss 5 mm or more) were 4 (20 ± 8.94%).
In order to determine the strength of predictor variables for development of chronic periodontitis in the studied contingent, the effect size of each variable was investigated separately. Non-parametric analysis (Fisher's test) was used. The odds ratio (OR) and the 95% confidence interval (CI) for each of the 12 variables were calculated.
The statistical analysis showed that the following variables were statistically non-significant (P>0.05) and they were excluded: smoking (P=0.349), alcohol use (P=0.151), vegetarian diet (P=0.162), diet (lack of fresh fruit and vegetables in the daily menu) (P=0.485), occupational hazards (P=0.080), oral hygiene (P=0.225), tooth clenching and grinding (P=0.081). The significant variables (P< 0.05) included: diabetes type 2, stress, crooked and overlapping teeth, dentist practices visits and calculus removal.
Multiple logistic regressions (Backward Conditional procedure) were used to assess the combined impact of the selected significant variables (via one-factor analysis). Following the multiple regression construct, three significant predictor variables were selected in the final equation: diabetes type 2, stress, crooked and overlapping teeth. These factors showed the strongest relative weight in the studied cohort. The variable calculus removal is not present in the final equation (P=0.800).
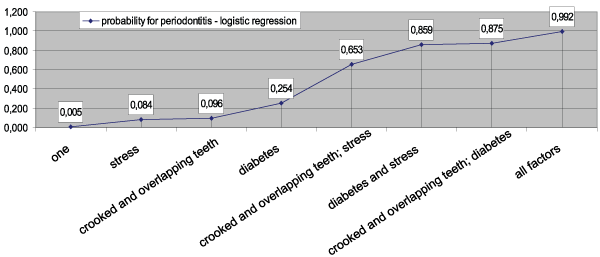
Based on the accumulated empirical data, a logistic model was constructed to assess the risk of periodontitis development. It incorporates the three selected variables: diabetes, stress, crooked and overlapping teeth. The model was shown to have a 93.80% predictive value (χ2=63.91; P=0.001) (Figure 1). Diabetes type 2 had the highest predictive value (the highest regression coefficient) [26].
After the significance of diabetes type 2 as a variable with a highest predictive value in the logistic model, the 228 sampled participants were interviewed. The analysis of the data shows the following:
Researchers believe that both types of diabetes (insulin and non-insulin dependent diabetes) are risk factors for periodontitis. The sampled participants were (14.91 ± 2.36%) stated they had diabetes type 2, which is a relatively high percentage.
The question of the significant link between the prevalence and severity of periodontal diseases among people with diabetes, their age and the presence of dental calculus [15], has often been discussed in the relevant publications. In this study, patients with diabetes in the age group 40-59 years (38.23 ± 8.33%) have the greatest relative share, followed by those over 60 years of age (35.29 ± 8.20%), while younger patients, aged 20-39 (26.47 ± 7.58%) have the smallest share (Table 1).
![]()
Table 1: Distribution of patients with diabetes by age in the sample
View Table 1
With regard to the removal of dental calculus, most of the surveyed - (58.82 ± 8.44%) stated that they had never had their dental calculus removed, while (41.18 ± 8.44%) had it removed on a regular basis (Table 2). The difference is statistically significant (χ2=31.88; P< 0.05) It proves the claim in the said publications that the presence of dental calculus in patients with diabetes exacerbates periodontal destruction.
![]()
Table 2: Dental calculus removal in the sampled patients with diabetes
View Table 2
Grossi et al. prove that diabetes, combined with smoking and the presence of periodontal pathogens P. Gingivalis and B. Forsythus, are the main clinical determinants of periodontal destruction [21]. This motivated the authors to investigate the prevalence of the harmful habit of smoking among the sampled people with diabetes (Table 3). It was established that most of them smoked (85.29 ± 6.07%).
![]()
Table 3: Prevalence of smoking among the sampled people with diabetes
View Table 3
The high result supports the opinion expressed by most researchers.
Limitation of the study: This study assesses the association between the diabetes type 2 and chronic periodontitis using the methodologies of case-control study. Further studies with data obtained from a prospective cohort design is necessary to confirm a cause-effect association - diabetes type 2 and chronic periodontitis in Bulgarien patients.
Conclusion
The prevalence of diabetes is growing rapidly worldwide, especially in developing nations. In our contingent, diabetes had a strongest predictive value in the development of the disease. Based on these findings, we recommend a closer cooperation between dental practitioners, endocrinologists and general practitioners, aimed at prevention, early detection and diagnosis of periodontal diseases.
Periodontitis is the second largest oral health problem, affecting 10-15% of the world's population, Taking these results into account, if dental practitioners are presented with the elaborated model for assessing the risk of developing chronic periodontitis, it will be possible, as early as upon taking the patient's history and in the presence of the factors of diabetes, stress, misshapen and overlapping teeth, to apply a more adequate prophylactic approach to periodontitis and the prevention of its development.
References
-
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103: 137-149.
-
Löe H (1993) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16: 329-334.
-
Van Dyke TE, Sheilesh D (2005) Risk factors for periodontitis. J Int Acad Periodontol 7: 3-7.
-
Stabholz A, Soskolne WA, Shapira L (2010) Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000 53: 138-153.
-
AlJehani YA (2014) Risk factors of periodontal disease: review of the literature. Int J Dent 2014: 182513.
-
Clerehugh V, Tugnait A, Genco R (2009) Periodontology at a Glance. Wiley-Blackwell.
-
Capilouto ML, Douglass CW (1988) Trends in the prevalence and severity of periodontal diseases in the US: a public health problem? J Public Health Dent 48: 245-251.
-
Sheiham A, Netuveli GS (2002) Periodontal diseases in Europe. Periodontol 2000 29: 104-121.
-
Van Dyke T (2010) Inflamation and diabetes. In: Di Annual World Dental Congress. Final Programme: 67.
-
Beck JD, Arbes SJ (2006) Epidemiology of gingival and periodontal diseases. In: Newman M, Takei H, Klokkevold P. Carranza "s Clinical Periodontology: 125-126.
-
Mealey BL (2006) Periodontal disease and diabetes. J Am Dent Assoc 137: 26S-31S.
-
Tervonen T, Oliver RC (1993) Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol 20: 431-435.
-
Westfelt E, Rylander H, Blohmé G, Jonasson P, Lindhe J (1996) The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol 23: 92-100.
-
Oliver RC, Tervonen T (1994) Diabetes--a risk factor for periodontitis in adults? J Periodontol 65: 530-538.
-
Ohlrich EJ, Cullinan MP, Leichter JW (2010) Diabetes, periodontitis, and the subgingival microbiota. J Oral Microbiol 2.
-
Sanz-Sanchez I, Bascones Martinez A (2008) Otras enfermedades periodontales. I: Periodontitis como manifestacion de enfermedades sistematicas. Av en Periodoncia 20: 59-66.
-
Navarro Sanchez AB, Almeida R, Bascones Martinez A (2002) Relacion entre diabetus mellitus y enfermedad periodontal. Av en Periodoncia 14: 9-19.
-
Sanz-Sanchez I, Bascones Martinez A (2009) Diabetus mellitus: Su implication en la patologia oral y periodontal. Av Odontoestomatol 25: 249-263
-
Khader YS1, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ (2006) Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications 20: 59-68.
-
Taylor JJ, Preshaw PM, Lalla E (2013) A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol Suppl 14: S113-S134.
-
Grossi SG, Genco RJ (1998) Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol 3: 51-61.
-
Southerland J, Taylor G, Offenbacher S (2005) Diabetes and periodontal infection: Making the connection. Clinical Diabetes 23: 171-178.
-
MacMahon B, Trichopulos D (1996) Epidemiology. Principles and Methods. Little, Brown and Company: 252.
-
Diabetes Atlas (2013) 6th edn. International Diabetes Federation: 7.
-
Borissova AM, Shinkov A, Kovatcheva R (2006) Prevalence of diabetes in Bulgaria and the significance of the risk factors: age, obesity and family history. Endocrinologia 7: 9.
-
Stoykova M, Musurlieva N, Boyadzhiev D (2014) Risk factors for development of chronic periodontitis in Bulgarian patients. Biotechnology and technological equipment 28: 1150.