International Journal of Diabetes and Clinical Research
Assessment of Circulatory Levels of Endothelin-1 in Diabetic Subjects Screened Through a Cross Sectional Study from Rural Women in Bangladesh
Md. Arifur Rahman1,2, Subrina Jesmin2,4*, Chishimba Nathan Mowa3, Farzana Sohael1,4, Junko Kamiyama2, AKM Ahsan Habib1, Nobutake Shimojo2, Md. Majedul Islam2-4, Masao Moroi5, Osamu Okazaki5, Naoto Yamaguchi6, Satoru Kawano2, Taro Mizutani2 and Yoshio Iwashima7
1Department of Cardiology, Shaheed Ziaur Rahman Medical College, Bangladesh
2Institiute of Clinical Medicine, Faculty of Medicine, University of Tsukuba, Japan
3Department of Biology, Appalachian State University, USA
4Health & Disease Research Center for Rural Peoples (HDRCRP), Bangladesh
5National Center for Global Health and Medicine (NCGM), Japan
6Center for Health Sciences, Ibaraki Prefectural University, Japan
7Division of Hypertension and Nephrology, Department of Medicine, National Cerebral and Cardiovascular Center, Japan
*Corresponding author:
Subrina Jesmin MD, Ph.D, Institiute of Clinical Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, 305-8575, Japan
Health & Disease Research Center for Rural Peoples (HDRCRP), Mohammadpur, Dhaka 1207, Bangladesh, Tel: +81-29-853-5633, Fax: +81-29-853-3092, E-mail: jsubrina@gmail.com
Int J Diabetes Clin Res, IJDCR-2-024, (Volume 2, Issue 1), Research Article; ISSN: 2377-3634
Received: November 30, 2014 | Accepted: February 24, 2015 | Published: February 26, 2015
Citation: Arifur Rahman MD, Jesmin S, Mowa CN, Sohael F, Kamiyama J, et al. (2015) Assessment of Circulatory Levels of Endothelin-1 in Diabetic Subjects Screened Through a Cross Sectional Study from Rural Women in Bangladesh. Int J Diabetes Clin Res 2:024. 10.23937/2377-3634/1410024
Copyright: © 2015 Arifur Rahman MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aims: Endothelin-1 (ET-1), a potential marker of endothelial dysfunction, has been shown to be elevated in diabetic mellitus (DM) subjects. However, to date, the circulatory profile of ET-1 and its association with DM have not been investigated in any South Asian country, including Bangladesh. The present study assessed circulating levels of ET-1 in subjects with or without DM and further examined the association of ET-1 with clinical and metabolic parameters in Bangladeshi rural women.
Main Methods: A total of 2022 rural Bangladeshi women were studied using a cross-sectional survey and then further analysis was performed based on a case control design between DM (n=195) and non-DM (204). Multiple regressions were used to examine the association between circulatory ET-1 level and DM.
Key findings: A total of 2022 rural Bangladeshi women aged ≥15 years were studied using a population-based cross-sectional survey (DM prevalence is 9.1%). ET-1 levels were significantly increased in diabetes subjects [DM vs. non-DM: 3.11 ± 0.16 vs. 1.97 ± 0.03, p< 0.001]. In multivariable analyses, after adjusting for age, ET-1was found to have significant positive association with waist circumference (p=0.029), fasting plasma glucose levels (p=0.001) and HDL-C (p=0.016). In multiple regression analysis considering ET-1 levels as a dependent variable, we found that plasma glucose levels and HDL-C are the independent determinants for plasma ET-1 levels in Bangladeshi rural women. Through tertile analysis, we found a significant increase in plasma levels of mean ET-1 as levels of blood glucose increased (p for trend< 0.001).
Significance: A higher circulatory concentration of ET-1 among DM subjects suggests possible endothelial dysfunction in this apparently healthy population. The relation between plasma ET-1 levels and DM needs further investigations aimed at defining the clinical predictive value of ET-1 in DM in the South Asian population.
Keywords
Diabetes mellitus, Endothelin-1, Rural women, Bangladesh, South Asia
Introduction
Diabetes Mellitus (DM) is a global epidemic affecting approximately 285 million people and has a high (and rising) prevalence in both developed and, more recently, developing countries [1]. DM has, in particular, become an important health concern in the South Asian region, with an estimated increase in the prevalence of diabetes of over 151% between the years 2000 and 2030.Specifically, recent epidemiological studies on South Asia have shown an increased prevalence of diabetes in India (12.1%) [2], Pakistan (11.1%) [3] and Bangladesh (7.9%) (rural population) [4]. Interestingly, this increase in prevalence has also been observed in South Asians in diasporanotably Asian Americans, and has been attributed to ageing of the population, urbanization, obesity and physical inactivity, and changing dietary habits [5].
Among various pathological complications, DM is normally associated with macro and micro vascular disorders. The primary aetiology responsible for diabetic vascular complications is endothelial dysfunction [6], which is characterized by an imbalance between endothelial-derived vaso-dilator and -constrictor substances, such as the vasoconstrictor factor, endothelin-1 (ET-1). Thus, ET-1 plays an important role in the pathogenesis of vascular complication in diabetes [7].
ET is a potent endothelial cell-derived venous and arterial vasoconstrictor peptide [8] that consists of three family members of 21 amino acid peptides (ET-1, ET-2, and ET-3) [9]. Among the family members ET-1 is the most abundant isoform and the most potent known vasoconstrictor in humans and contributes to the maintenance of basal vascular tone [10]. ET-1 is also the principal cardiovascular isoform of the endothelial system [8]. Two receptor subtypes, endothelin A- and B- receptors (ETAand ETB), mediate the effects of ET-1. Vascular smooth muscle cells express both ETA and ETB, while endothelial cells express primarily ETB [11]. On smooth muscle cells, ETA mediates vasoconstriction and mitogenesis, while ETB receptor has a dual function and has been shown to cause both vasoconstriction and vasodilatation [12,13]. ETA receptor activation contributes to coronary constrictor tone and peripheral and endothelial dysfunction [14,15]. In isolated human internal mammary and porcine coronary arteries, ETB receptor mediates ET-1-induced vasoconstriction [13]. In healthy humans, selective ETB receptor antagonism increases peripheral vascular resistance, implying that vasodilation is the dominant (“favoured”) ETB pathway [16]. However, the balance between vasodilator and vasoconstrictor ETB pathways may be altered in pathological conditions [17,18]. For instance, increased ET-1 levels have been detected in hypertension, coronary artery disease, heart failure, metabolic syndrome and DM. ET-1 plasma levels are also increased in obese patients with metabolic syndrome [19]. Chronic exposure of ET-1 leads to insulin receptor desensitization and the vasoconstrictor tone of ET-1 is enhanced in patients with type 2 DM [20].
To our knowledge, to date only a few studies have investigated the association between ET-1 and DM in apparently healthy population, and most of these (previous) studies have largely focused on clinical patients. Moreover, no previous studies have been conducted on South Asian populations or populations from low income countries. Since racial differences are known to exist in patterns of plasma ET-1 levels, it is essential that more research that address the association between ET-1 and DM within a specific or defined ethnic or racial context, be performed.
Thus, here our aim was to examine the association between circulating ET-1 levels and DM among Bangladeshi rural women.
Materials and Methods
Study procedure
This study is a community-based cross-sectional study performed in women from rural Bangladesh. A total of 2022 females aged ≥15 years were selected using the stratified multistage random sampling. This sample size was sufficient to test all our formulated research hypotheses at the 5% level of significance, with a power of 80% (β=0.20). We used the World Health Organization’s (WHO) STEPS approach (modified), which entails a stepwise collection of the risk factor data, based on standardized questionnaires covering demographic characteristics, somatic illnesses, somatic and mental symptoms, medications, life style, and health-related behaviour (step 1), basic physical measures (step 2) and basic biochemical investigations, such as levels of blood glucose and cholesterol (step 3). The women were recruited from twelve village communities located in Gaibandha district. The respondents were selected randomly after selecting upazila and villages, and were recruited through local announcements in the communities of interest and by house-to-house visits. The details of the study area have been described before in our previous study [21]. The data from participants were obtained through interviews and clinical examinations at mobile health examination centres, where blood samples were also collected. The study was approved by the Ethical Committee of the Health and Disease Research Center for Rural Peoples (HDRCRP), Dhaka, Bangladesh, Shahid Ziaur Rahman Medical College, Bogra, Bangladesh and Institute of Clinical Medicine, University of Tsukuba, Japan and conforms to the principles outlined in the Helsinki Declaration. All participants gave their written informed consent prior to inclusion in the study.
Study subjects
Out of 2108 women, we excluded the following subjects: women with chronic illness, such as hypothyroidism; pregnant women; those on hormone replacement therapy (HRT); as well as women with known illness, such as ischaemic heart disease (IHD). After the exclusion, a total of 2022 subjects remained in this study. Among these 2022 subjects, 195 diabetic cases and 204 non-diabetic cases were used for further analysis in a case control design.
Anthropometric and other variables
Anthropometric measurements on individuals wearing light clothing and without shoes were conducted by well-trained examiners, as described here: height was measured to the nearest 0.1cm using the portable stadiometer; weight was then measured in an upright position, to the nearest 0.1kg, using a calibrated balance beam scale; BMI was calculated as body weight (kg) divided by the square of the body height (m2); and waist circumference measurements were taken at the end of normal expiration, to the nearest 0.1cm, by measuring from the narrowest point between the lower borders of the rib cage and the iliac crest. Blood pressure was measured twice in the right arm in a sitting position using the standard mercury manometer and cuff, to the nearest 2 mmHg, with the initial reading taken at least 5 minutes after the subject was made comfortable, and again after an interval of 15 minutes. The average systolic and diastolic blood pressures were then estimated.
Biochemical analysis
Blood for biochemical analysis was obtained from the participants after a 10-12 hour overnight fast. The blood sample was collected using the standard blood sample collection procedure and both serum and plasma were processed. Triglycerides levels were measured by lipoprotein lipase method (Wako Chemicals, Tokyo, Japan), HDL cholesterol was measured with the Determiner-L kit (Kyowa Co Ltd, Tokyo, Japan). Fasting plasma glucose levels were measured with the Hexokinase G-6-PDH kit (Wako Pure Chemical Industries Ltd, Osaka, Japan). Plasma levels of ET-1 were measured using specific commercial enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s directions (Quantikine, R & D systems, Minneapolis, MN, USA). Oral glucose tolerance test was performed in those subjects having fasting plasma glucose levels more than 7mmol/l.
Statistical analysis
Differences in clinical characteristic between DM and non-DM subjects were assessed by t-test and the Mann-Whitney test for normal and skewed continuous variables, respectively. Mean ± S.E is presented. BMI, fasting plasma glucose, triglycerides, insulin, and ET-1 were log transformed to better approximate normal distribution. Multiple linear regression analysis was used to evaluate the association between ET-1 plasma levels and clinical/metabolic parameters for unadjusted and age-adjusted model. To identify independent determinants of ET-1, stepwise multiple regression analysis was performed in forward direction with significance level for addition to the model set to 0.20. P values of less than 0.05 were considered statistically significant. All analyses were performed using Stata version 12.0 (StataCorp, College Station, Texas USA).
Results
The prevalence of DM in this study population was 9.1% in a sample size of 2022. Among these subjects, we conducted a case control study of subjects that were divided into two groups, namely DM and non-DM. The subjects with fasting blood glucose of more than 7mmol/l, which was later confirmed by oral glucose tolerance test (OGTT), were placed into the DM group. It should be noted that these DM subjects were selected from an initial cross sectional study survey conducted in apparently healthy rural women in Bangladesh. These DM subjects were diagnosed DM during the current study and never knew that they had diabetes prior to this study. The condition of these DM subjects was detected during the non-communicable disease screening conducted earlier in the study.
Table 1 shows clinical characteristics of subjects based on their diabetic status, i.e., DM and non-DM. Generally, DM subjects were a bit older. Mean values of waist circumference, fasting plasma glucose, and HOMA-IR were significantly higher in DM subjects, whereas levels of HDL were significantly lower in DM subjects compared to non-DM.
![]()
Table 1: Clinical characteristics of subject according to diabetic status
View Table 1
As shown in the figure 1, levels of plasma ET-1 were significantly higher in DM subjects than in non-DM subjects.
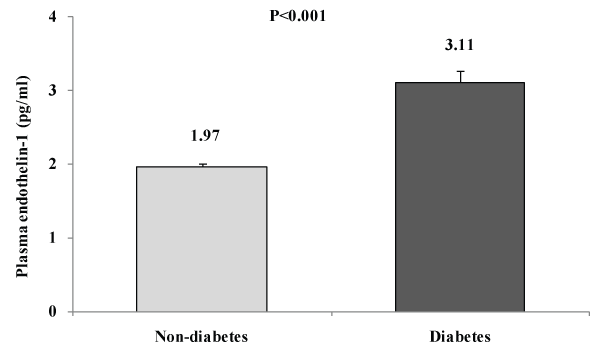
Figure 1: Mean plasma endothelin-1 levels according to diabetic (DM) and
non-diabetic (non-DM) statuss
View Figure 1
Table 2 shows the association between plasma levels of ET-1 and metabolic or other clinical parameters. In multivariable analyses, after adjusting for age, ET-1 had significant positive association with waist circumference (p=0.029), fasting plasma glucose levels (p=0.001), and HDL-C (p=0.016).Other variables, including BMI, triglycerides, blood pressure, total cholesterol, and insulin levels were not associated with ET-1 levels. Stepwise multiple regression analysis, considering all the variables presented in Table 1, revealed that fasting plasma glucose and HDL levels were independent determinants of plasma ET-1 levels (Table 3).
![]()
Table 2: Association between endothelin-1 and others parameters
View Table 2
![]()
Table 3: Multiple linear regression of endothelin-1 and others predictors
View Table 3
Figure 2 shows the association between plasma ET-1 levels and tertile of blood glucose. As shown in figure 2, mean ET-1 levels significantly increase as levels of blood glucose increases (p for trend< 0.001).
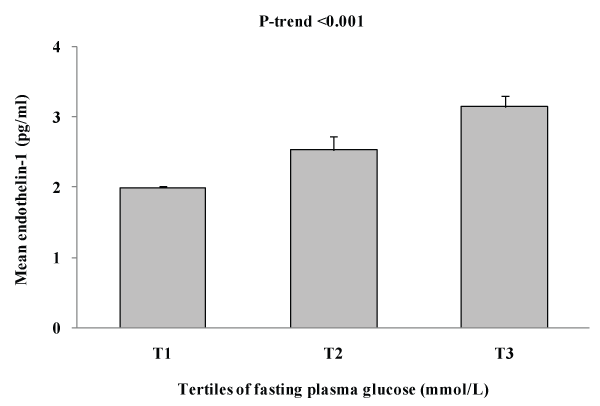
Figure 2: Shows the association between plasma ET-1 levels and tertile of
fasting plasma glucose
View Figure 2
Discussion
To our knowledge, this is the first study to address an association between circulating levels of ET-1 and DM in a Bangladeshi group. In this cross-sectional study of Bangladeshi rural women, we found that plasma ET-1 levels were significantly higher in subjects with DM than without DM. Among the cardiometabolic risk factors, plasma glucose and HDL levels show significant positive association with ET-1.
The important role of ET-1 as a modulator of vascular tone and growth makes it a vital potential predictive indicator of diabetic vascular complications [22]. It has been shown that levels of plasma ET-1 during a diabetic state were elevated in both animal models [23] and human patients [24,25]. The elevated ET-1 levels among subjects with DM and the significant positive association between ET-1 and DM in our study is consistent with data from many previous studies [24-26]. In particular, ET-1 levels were significantly higher in subjects with diabetes type 2 than non-diabetes [27]. Both type 2 diabetes and metabolic syndrome are associated with highly variable levels of ET-1 in postmenopausal women, and diabetic women had significantly elevated ET-1 levels [28]. In addition, the balance between nitric oxide (NO) (vasodilator) and ET-1 (vasoconstrictor) is disrupted in experimental conditions that simulated hyperglycemia, as well as in high free fatty acid (FFA) levels observed in diabetes [29]. Notably, is the fact that the current population studied here were apparently healthy, in contrast to the majority of previous studies that used known diagnosed diabetic, obese, or patients with heart disease. For this reason, the current study population had no prior treatment for neither diabetes nor diet restriction as they did not know that they had diabetes. Again, the present data is consistent with a previous study reporting that ET-A receptor was sensitized in pre-diabetic dogs [30] and ET-1 mediated vasoconstrictor tone was elevated in people with impaired fasting blood glucose [31]. Thus pre-diabetes mellitus, which is characterized as impaired fasting blood glucose may be strongly associated with ET-1. Endothelial vasomotor dysfunction in early diabetes rats might be related to increasing vessel stress, ET-1 production, and compensating the increase of NO [32].
Multiple regression analysis of the current study shows a significant association between plasma ET-1 levels and fasting plasma glucose and HDL levels. Also we found that mean value of ET-1 increases with the increase in the levels of fasting plasma glucose. Hyperglycaemia, the primary metabolic disturbance of diabetes, has been shown to increase [33] the release of ET-1 from endothelial cells in culture. Importantly, it was shown that altered plasma ET-1 in diabetes can be restored to normal levels by restoration of metabolic control [34]. Thus, elevated plasma glucose levels may contribute to the increase of ET-1 levels in diabetic subjects as observed in the current study population. In addition, the current study reveals that ET-1 levels have a negative significant association with plasma HDL levels. In fact, previous in vitro studies have demonstrated that HDLs exert anti-atherogenic effects by inhibiting ET-1 release in a polar manner [35] in human endothelial cells. The other novel finding of the current study through multiple regression analysis is that ET-1 has significant positive association with age. Aging is associated with elevation of ET-1 levels (mRNA and protein) in plasma and vascular tissues [36-38], which correlates well with vascular tone or endothelium-dependent vascular dilation. However, to date, no correlation in diabetic patients between ET-1 levels and age, sex, fasting blood glucose, Dyslipidemia, C-peptide, BMI, and smoking has been observed by previous studies [39]. The current data implies that plasma ET-1 levels in diabetic subjects are differentially correlated with socio-demographics and the clinical parameters in a context specific manner. In addition, we also checked level of inflammatory cytokine like tumor necrosis factor (TNF)-alpha in current study design and found no significant difference in TNF-alpha level in between DM and non-DM subjects in our present study population (Arifur et al., unpublished observation, 2015).
Although significant positive association between pro-ET-1 and high blood pressure has been observed in previous study [40], in the current study we did not find any significant association between ET-1 levels and blood pressure. Further, even though plasma ET-1 increased in Type II diabetes, those with both hypertension and macrovascular disease, but not with hypertension alone, had higher plasma ET-1 than those with uncomplicated Type II diabetes [41,42]. Using an apparently healthy population, Hirai et al., found significant positive association between ET-1 and systolic and diastolic blood pressure in univariate analysis. However, in multiple stepwise regressions model both systolic and diastolic blood pressures were not associated with ET-1 [43]. Similarly, Piatti et al., found no significant positive association of ET-1 with systolic and diastolic blood pressure in multiple regression analysis [44]. Differences in background characteristics of the study population as well as the duration of diabetes may be a potential reason for the inconsistent findings between the two studies.
Previously it has been shown that diabetic disease duration of 10 years or more has significant effect on plasma ET-1 levels [39].During this period, the incidence and grade of the microvascular complications increase, and ET-1 levels are elevated [39]. However, Vertello et al. reported no correlation between duration and ET-1 levels [45]. Based on the current data, no comment can be made on the duration of diabetes and its correlation with the levels of plasma ET-1 levels since we did not initially know that the DM group study subjects had diabetes.
The major strengths of the present study are use of a large community-based survey, with a relatively large sample size, and also the comprehensive analysis of plasma ET-1 levels as well as other important cardio metabolic risk factors in diabetic and non-diabetic subjects. However, the current study has a number of limitations that need to be highlighted. Firstly, existence of an association in a cross-sectional study does not necessarily indicate causality. Secondly, each biomarker was sampled and evaluated only once in each participant. Ideally, there should be more than one sampling and evaluation per participant at different time-points. Beside, we were not able to adjust for important lifestyle risk factors, including smoking and alcohol drinking, in order to assess the association between ET-1 and cardio-metabolic risk factor. However, because alcohol drinking and smoking are very uncommon among women in Bangladesh, these variables were most likely non-existent in our subjects. And finally, only rural women from a lower socioeconomic class were enrolled, thus limiting generalization of the data obtained to the whole adult female population in Bangladesh.
Conclusion
The present study is the first comprehensive approach using a large sample size to establish the association between circulatory levels of ET-1 and DM in the context of a South Asian country, namely Bangladesh. We also show here that circulatory levels of ET-1 were significantly higher in diabetic than in non-diabetic subjects. Among the cardio metabolic risk factors, fasting plasma glucose, and plasma HDL showed significant association with ET-1. Further prospective studies are needed to elucidate the role of ET-1 in subjects with DM and its associated disorders.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (overseas academic) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23406037, 23406016, 23406029, 24406026, 25305034), and Japan Society for the promotion of Science. Current project (WDF11-610) on gestational diabetes from World Diabetes Foundation (WDF), Denmark to HDRCRP has also supported a part of this work.
References
-
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053.
-
Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, et al. (2001) High prevalence of diabetes and impaired glucose tolerance in India: national urban diabetes survey. Diabetologia 44: 1094-1101.
-
Shera AS, Rafique G, Khwaja IA, Baqai S, Khan IA, et al. (1999) Pakistan National Diabetes Survey prevalence of glucose intolerance and associated factors in North West at Frontier Province (NWFP) of Pakistan. J Pak Med Assoc 49: 206-211.
-
Bhowmik B, Afsana F, My Diep L, BinteMunir S, Wright E, et al. (2013) Increasing Prevalence of Type 2 Diabetes in a Rural Bangladeshi Population:A Population Based Study for 10 Years. Diabetes Metab J 37: 46-53.
-
McNeely MJ, Boyko EJ (2004) Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 27: 66-69.
-
Wheatcroft B, Williams IL, Shah AM, Kearney MT (2003) Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 20: 255-268.
-
Kalani M (2008) The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc Health Risk Manag 4: 1061-1068.
-
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, et al. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411-415.
-
Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, et al. (1989) The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA 86: 2863-2867.
-
Haynes WG, Webb DJ (1994) Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet 344: 852-854.
-
Molenaar P, O'Reilly G, Sharkey A, Kuc RE, Harding DP, et al. (1993) Characterization and localization of endothelin receptor subtypes in the human atrioventricular conducting system and myocardium. Circ Res 72: 526-538.
-
Sakurai T, Morimoto H, Kasuya Y, Takuwa Y, Nakauchi H, et al. (1992) Level of ETB receptor mRNA is down-regulated by endothelins through decreasing the intracellular stability of mRNA molecules. Biochem Biophys Res Commun 186: 342-347.
-
Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF (1994) Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 89: 1203-1208.
-
Kyriakides ZS, Kremastinos DT, Kolettis TM, Tasouli A, Antoniadis A, et al. (2000) Acute endothelin-A receptor antagonism prevents normal reduction of myocardial ischemia on repeated balloon inflations during angioplasty. Circulation 102: 1937-1943.
-
Halcox JP, Nour KR, Zalos G, Quyyumi AA (2001) Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res 89: 969-976.
-
Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, et al. (1999) Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension 33: 581-585.
-
Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO 3rd, Panza JA (1999) Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension 33: 753-758.
-
Pernow J, Böhm F, Johansson BL, Hedin U, Rydén L (2000) Enhanced vasoconstrictor response to endothelin-B-receptor stimulation in patients with atherosclerosis. J Cardiovasc Pharmacol 36: S418-S420.
-
Ferri C, Bellini C, Desideri G, Baldoncini R, Properzi G, et al. (1997) Circulating endothelin-1 levels in obese patients with the metabolic syndrome. Exp Clin Endocrinol Diabetes 105: 38-40.
-
Cardillo C, Campia U, Bryant MB, Panza JA (2002) Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783-1787.
-
Jesmin S, Akter S, Rahman MM, Islam MM, Islam AM, et al. (2013) Disruption of components of vascular endothelial growth factor angiogenic signalling system in metabolic syndrome. Findings from a study conducted in rural Bangladeshi women. Thromb Haemost 109: 696-705.
-
Hopfner RL, Gopalakrishnan V (1999) Endothelin: emerging role in diabetic vascular complications. Diabetologia 42: 1383-1394.
-
Takeda Y, Miyamori I, Yoneda T, Takeda R (1991) Production of endothelin-1from the mesenteric arteries of streptozotocin–induced diabetic rat. Life Sci 48: 2553-2556.
-
Takahashi K, Ghatei MA, Lam HC, O’Halloran DJ, Bloom SR (1990) Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia 33: 306-310.
-
Collier A, Leach JP, Maclellan A, Jardine A, Morton JJ, et al. (1992) Plasma endothelin-like immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care 15: 1038-1040.
-
Pernow J, Shemyakin A, Böhm F (2012) New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 91: 507-516.
-
Hermans MP, Ahn SA, Rousseau MF (2009) Raised natriuretic peptides, big-endothelin-1 and improved beta-cell function in type 2 diabetic males with hyperuricaemia. Diab Vasc Dis Res 6: 190-193.
-
Saltevo J, Puolakka J, Ylikorkala O (2000) Plasma endothelin in postmenopausal women with type 2 diabetes mellitus and metabolic syndrome: a comparison of oral combined and transdermal oestrogen-only replacement therapy. Diabetes Obes Metab 2: 293-298.
-
Storniolo CE, Roselló-Catafau J, Pintó X, Mitjavila MT, Moreno JJ (2014) Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol 2C: 971-977.
-
Knudson JD, Rogers PA, Dincer UD, Brats IN, Araiza AG, et al. (2006) Coronary vasomotor reactivity to endothelin-1 in the prediabetic metabolic syndrome. Microcirculation 13: 209-218.
-
Diehl KJ, Templeton DL, Ma J, Weil BR, Greiner JJ, et al. (2013) Impaired fasting blood glucose is associated with increased endothelin-1 vasoconstrictor tone. Atherosclerosis 229: 130-133.
-
Wang JS, Yin HJ, Guo CY, Huang Y, Xia CD, et al. (2013) Influence of high blood glucose fluctuation on endothelial function of type 2 diabetes mellitus rats and effects of Panax Quinquefolius Saponin of stem and leaf. Chin J Integr Med 19: 217-222.
-
Yamauchi T, Ohnaka K, Takayanagi R, Umeda F, Nawata H (1990) Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett 267: 16-18.
-
Hopfner RL, Misurski D, Wilson TW, McNeill JR, Gopalakrishnan V (1998) Insulin and vanadate restore decreased plasma endothelin concentrations and exaggerated vascular responses to normal in the streptozotocin diabetic rat. Diabetologia 41: 1233-1240.
-
Fan J, Unoki H, Iwasa S, Watanabe T (2000) Role of endothelin-1 in atherosclerosis. Ann N Y Acad Sci 902: 84-93.
-
Goettcsh W, Lattman T, Amann K, Szibor M, Morawietz H, et al. (2001) Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun 280: 908-913.
-
Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, et al. (2003) Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol 95: 336-341.
-
Ishihara A, Katano Y (2006) Investigation of differentially expressed genes in the ventricular myocardium of senescent rats. Ann NY Acad Sci 1067: 142-151.
-
Ak G, Buyukberber S, Sevinc A, Turk HM, Ates M, et al. (2001) The relation between plasma endothelin-1 levels and metabolic control, risk factors, treatment modalities, and diabetic microangiopathy in patients with Type 2 diabetes mellitus. J Diabetes Complications 15: 150-157.
-
Seissler J, Feghelm N, Then C, Meisinger C, Herder C, et al. (2012) Vasoregulatory peptides pro-endothelin-1 and pro-adrenomedullin are associated with metabolic syndrome in the population-based KORA F4 study. Eur J Endocrinol 167: 847-853.
-
Fernandez AC, Patino R, Ibarra J, Fernandez L, Roldan M, et al. (1995) Endothelin in hypertension and diabetes mellitus. In: Luscher TF (ed) Endothelin in cardiovascular diseases. Springer, Berlin Heidelberg, New York: 157-170.
-
Perfetto F, Tarquini R, de Leonardis V, Piluso A, Becucci A,et al. (1997) Vascular damage and not hypertension per se influences endothelin-1 plasma levels in patients with non insulin dependent diabetes mellitus. Recenti Prog Med 88: 317-320.
-
Hirai Y, Adachi H, Fujiura Y, Hiratsuka A, Enomoto M, et al. (2004) Plasma endothelin-1 level is related to renal function and smoking status but not to blood pressure: an epidemiological study. J Hypertens 22: 713-718.
-
Piatti PM, Monti LD, Galli L, Fragasso G, Valsecchi G, et al. (2000) Relationship between endothelin-1 concentration and metabolic alterations typical of the insulin resistance syndrome. Metabolism 49: 748-752.
-
Bertello P, Veglio F, Pinna G, Gurioli L, Molino P, et al. (1993) Plasma endothelin in Type 2 diabetes mellitus patients with and without complications. Diabetes Care 17: 574-577.





