International Journal of Diabetes and Clinical Research
The Role of Netrin-1 in Diabetic Retinopathy: A Promising Therapeutic Strategy
Wenyi Wu and Luosheng Tang*
Department of Ophthalmology, Second Xiangya Hospital, Central South University, China
*Corresponding author:
Luosheng Tang, Department of Ophthalmology, Second Xiangya Hospital, Central South University, Changsha, Hunan 410008, China, Tel: 0731-85295838; E-mail: tangls57@gmail.com
Int J Diabetes Clin Res, IJDCR-1-008, (Volume 1, Issue 2), Review Article; ISSN: 2377-3634
Received: October 08, 2014 | Accepted: November 01, 2014 | Published: November 06, 2014
Citation: Wu W, Tang L (2014) The Role of Netrin-1 in Diabetic Retinopathy: A Promising Therapeutic Strategy. Int J Diabetes Clin Res 1:008. 10.23937/2377-3634/1410008
Copyright: ©2014 Wu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Netrin-1 which belongs to axon guidance cue is necessary for neural and vascular development. It involves in regulating axon guidance for attraction or repulsion and it has a dual function in endothelial tip cell migration during angiogenesis. Many evidences have proved that netrin-1 plays an important role in angiogenesis, cancer progression and inflammation disease. Here we provide a comprehensive review on the role of netrin-1 in retinal and angiogenesis developments .What’s more, some associated signaling pathways in diabetic retinopathy are also discussed. Overall, the current available data suggests that the netrin-1 could be a promising target for the development of anti-angiogenesis drugs.
Keywords
Guidance cues, Netrin-1, Angiogenesis, Diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is the largest contributor to the low quality of life in diabetes, which affects approximately 80% of diabetes who have suffered for more than 10 years. DR remains the most common origin for irreversible vision loss through macula edema to vitreous hemorrhage in developed countries. Over accumulation of glucose damages the tiny blood and increases inflammation factor Prostaglandin E2 (PGE2) by activating NF-Kb [1] . And patients with developing peripheral and autonomic neuropathy possess a higher risk of retinopathy.
Netrin-1 is one kind of the four main guidance molecules which was purified by Tessier-Lavigne in screening for proteins that regulated neural development [2] . It stimulates angiogenesis and guides the vessel to follow very precise path way. This discovery launched a scientific race to identify the relation of nerves and blood vessels and suggested that there may be molecular crosstalk and common cues between nervous and angiogenesis [3, 4] . However, despite the importance of this intimate relationship, how the guidance molecular signals stimulate intracellular pathway in angiogenesis remains mystery. Understanding the molecular mechanisms of how neural and vascular network coordinately communicate needs further studies.
Recently, many scientists has turned their eyes on neuropathy in diabetic retinopathy [5] , Since it was proved that neurodegeneration is an early event in the pathogenesis of DR [6]. Thus, it is not surprising whether guidance molecular contributes to DR. So we summarize the current state of researches on netrin-1 in the development of retinal vessel and focus on its participation in angiogenesis. In addition, the possibility to target these molecules as a therapeutic approach in DR is also discussed.
Netrin-1 and their Receptors
Netrin-1 was first described in the nematode Caenorhabditis elegans in 1996 [2]. It belongs to a family of extracellular matrix proteins that present structural similarity with the laminin. Three Netrins (Netrin-1//3/4) and two membrane proteins related GPI-linked, netrin-G1 and G2 have been found in vertebrates. Netrin-2 was only found in the mouse. The different ligands are summarized in table 1. They are laminin-related proteins with an N-terminal laminin VI domain, a central laminin V domain and a C-terminal domain. Netrin-1 has been linked to two families: Deleted in Colon Cancer (DCC) family, including DCC and neogenin, and UNC5s homologue family. In addition, the membrane-associated adenosine A2b receptor, down syndrome cell adhesion molecule (DSCAM), several integrins (a6ß3 and a3ß1) and cerebellin-4 also act as direct receptors for netrin-1 or as co-receptors with DCC [7,8]. Different receptors can mediate different pathways which activate different biological effects.
![]()
Table 1: Different netrins and their receptors
View Table 1
DCC and UNC5 are single-pass transmembrane receptors that possess extracellular immunoglobulin domains. The DCC extra¬cellular domain contains four immunoglobulin-like domains and six fibronectin type III-like repeats. Its cytoplasmic domain includes an addiction dependence domain (ADD). UNC5 contain two thrombospondin-like repeats in the extracellular region, a zonula occludens-1 and a death domain in the intracellular region [9] (Figure 1). It is generally supposed that cell-surface membrane receptors initiate signal transduction upon binding to cognate ligands and are silent when not ligated. But it doesn’t apply to UNC5 and DCC receptors, which are known as dependence receptors [10]. UNC5B is a dependence receptor which is known to induce apoptosis in the absence of netrin-1, while transducing a cell survival signal when engaged by the ligand. DCC and UNC5 intracellular region can be cleaved by caspase3,which can induce apoptosis signaling [11].
![]()
Table 2: Netrin-1’s different function on neurology.
View Table 2
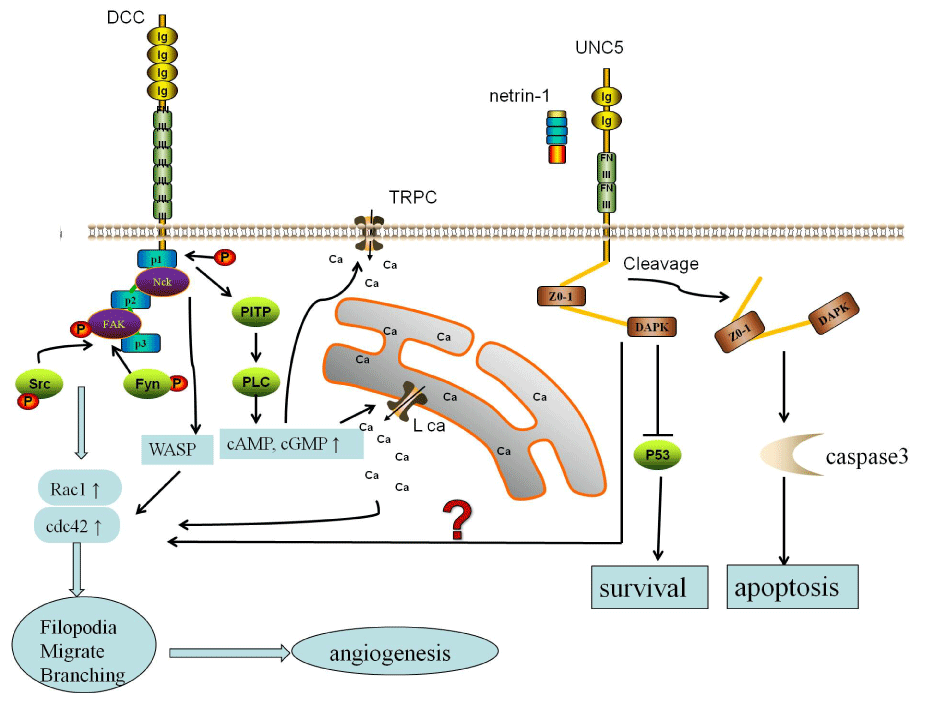
Figure 1: Structure and angiogenesis signal of unc5 and DCC
The DCC extra¬cellular domain contains four immunoglobulin-like domains
and six fibronectin type III-like repeats. UNC5 receptors contain two
thrombospondin-like repeats in the extracellular region, and a zonula
occludens-1 and a death domain in the intracellular region. There are three
pathways in netrin-1 of angiogenesis cascade. first pathway, the focal
adhesion kinase (FAK) recruit phosphorylation of Src and Fyn, and lead to
an increase in Rac1 and Cdc42. In second pathway, phosphatidylinositol
transfer protein a (PITP) induces phospholipase C (PLC) to increase the ratio
of cAMP and cGMP. Then activation L-type Ca2+ channels as well as transient
receptor potential channels (TRPC’s). And this increased calcium can activate
of Rho GTPases, Cdc42 Rac1. The last possible pathway of netrin is NcK
and Wiskott–Aldrich syndrome protein WASP triggers Rac1 and Cdc42 and
subsequently axonal growth.
View Figure 1
DCC’s intracellular domain associates with other partners, consisting of focal adhesion kinase (FAK), the non-catalytic region of tyrosine kinase (Nck1) and phosphatidylinositol transfer protein a (PITPa). UNC5 is a p53 target gene but netrin-1 binding inhibits p53-induced apoptosis [12] (Figure 1). DCC and UNC5 can form homodimers or multimers. This multimerization was shown to be sufficient to inhibit apoptosis signal [13]. More importantly multiple pathways are also activated by netrin-1, including MAPKs, PKC, PI3K-Akt, ERK-1/2 , small GTPase Rac-1/Cdc-42 and so on [8]. One consequence is the regulation of cell motility, invasion and morphogenesis of the vascular system through cytoskeletal rearrangements. Many these pathways may lay foundation in its dual function on angiogenesis.
Netrin-1 signaling is more complex. We have known that Netrin-1 can mediate attraction or repulsion depending on the receptor they bind [10]. Binding of Netrin-1 to DCC induces axonal attraction, whereas binding to the UNC5 family cause repulsion. But other evidences have proved that DCC can mediate repulsion through associating with of DCC and UNC5 in the cytoplasm.Netrin-1 not only regulates axon guidance, but also orientates cell migration in the developing vessel system [14]. Indeed, studies have found that netrin-1 was synthesized by swine follicular cells and secreted in the follicular fluid where it exerts regulatory effects on vascular development [15]. Overall, Netrin-1 is widely expressed outside the nervous system including endothelial cell, arterial smooth muscle cell and retina where they may play an important role in branching, morphogenesis of endothelial cell in DR.
Netrin-1 in Retina Development
It has been demonstrated that the guidance of retinal ganglion cell (RGC) axons go through the optic disc and even optic tectum is dependent on the Netrin-1/DCC axon guidance system [16,17]. What’s more, mRNA and protein of DCC were highly expressed in the early retina when RGC migrated towards the emerging layer and down-regulation on the distal axonal segments within the optic nerve. These indicate that netrin-1/DCC play an guidance role in the migration of newly born neurons within the developing retina [18]. To gain further insight into Netrin-1 and its receptors in retinal development ,Shi M and his colleges had found that the DCC(-/-) or lacking the intracellular P3 domain of DCC receptor causes a significant loss of RGCs and displaced amacrine cells ,but do not involve with other cell types in retinas. So ,their findings also suggest that DCC is a key regulator for the survival of specific types of neurons during retinal development and that DCC-P3 domain is essential for this developing event [19]. In addition, Netrin-1’s family member Netrin-G2 (NGL-2),a central component of pathway-specific development in the outer retina, can assemble pre-synaptic ribbons of rods and regulates pre-synaptic maturation [20]. Thus, netrin-1 is essential to retina development.
It is widely believed that the nervous and vascular systems are both exquisitely branched and complicated systems and their proper development require careful guidance. So we can hypothesize that netrin-1 may also play an important role in the development of retina vessel and it has been proved in sympathetic arterial [21]. During eye development, retinal vessels form three vascular plexuses in a highly reproducible manner, leading to the formation of distinct vascular. For example, astrocyte interacts with RGCs via PDGF-A/PDGFR-a to provide a template for vascularization, and tip cell guide the vessel toward the retinal periphery under the VEGF signaling and some axon guidance factors, such as semapherins and netrins [22,23].
Researches have showed that netrin-1 can control morphology of endothelial cells and vascular smooth muscle cells. Increased level of netrin-1 in murine retina under hypoxia may be a key factor in retinal neovascularization [24] .They are implicated in the reorganization of the cytoskeleton, as well as endothelial tip cell migration in retinal development and tumor [25]. What’s more, Netrin’s receptor UNC5B is highly expressed at postnatal day 0 (P0) of retinal vascular development and their localization become restricted to arteries, capillaries, and endothelial tip cells of retinal vessels during the period of active angiogenesis (P0 until P8–P9), and became progressively down-regulated once angiogenesis of these vessels have ceased. At P12, expression was observed in arteries, but extinguished in the most capillaries. After P21, reporter staining in retinal vessels became undetectable [26]. So with this study we can conclude that netrin-1/UNC5B can activate retinal vessel development and the fluctuation of UNC5B expression in vascular may affect the migration of tip cell, even stimulate the cytoskeletal protein in tip cell to pro-angiogenesis. However, it is contradict to the study conducted by Lu in 2004 [27], who thought the role in angiogenesis was suppression. So it still needs more research to indentify its effect on vessels.
Netrin-1 in Angiogenesis
The nervous and vascular system share similarities at the anatomical and cellular level, it was asked if axon guidance cues, such as netrin-1, pattern vasculature growth by direct interaction with endothelial cell surface receptors. UNC5B, predominantly endothelial-expressed receptor, is largely confined to the vasculature, with only few expression sites on other tissues, thus suggesting a broader role of these molecules in processes of angiogenesis. Even though abundant literature reported both pro-angiogenic and anti-angiogenic activities, the precise angiogenic roles of netrin-1 remains unclear [27-30].
Netrin-1, a laminin-related secrete protein that firstly described as a guidance cue during neurogenesis, act as a dual function in angiogenesis [31]. It was thought to be a protect factor as its repulsive effect on vessel and anti-inflammation factors through inhibits leukocyte migration in vitro and in vivo [32]. Genetic inactivating netrin-1 receptor UNC5B caused increased angiogenesis, suggesting its potential role as an anti-angiogenic growth factor [27,33]. UNC5B activation by Netrin-1 results in filopodial retraction of endothelial tip cells and inhibits neovessel sprouting processes such as bFGF induced matrigel plug invasion. Studies conducted by Larrivee have testified that UNC5B cytoplasmic domain was essential to repulsion requires signaling [26]. But latter evidence was demonstrated that it may promote angiogenesis in specific vascular beds [28]. This dual roles have indeed been proven for members of the three others families of guidance cues, namely Semaphorins, Ephrins and Slits proteins [34]. Netrin-1’s dual effect on angiogenesis may be caused by the following reasons: 1. Different receptors can mediate discrepancy biology effects 2. Different concentrations of netrin-1 present reverse effect on angiogenesis 3.the survival and apoptosis signal of the dependence receptor may have some relation with angiogenesis.
Netrin-1 has different receptors. UNC5B functions as repulsive receptor of netrin-1 in endothelial cells, restrains filopodia of the tip cell in morphogenesis of the vascular system [27]. While netrin-1 acts as pro-angiogenic factors and can remodel vascular in adult brain when bonding with DCC receptor [35,36]. These receptors can be found in distinct tissues. So the contradictory outcome of netrin-1 on vessel may be due to bonding with different receptors. But we have known that unc5b was highly expressed on tip cell. It didn’t explain its inhibitant role in angiogenesis as increasing expression on tip cell. So there must be other pathway involved in netrin-1.
Everything has two sides. Things will develop into the opposite direction when they become extreme. It has been generally admitted that netrin-1’s physiological concentration ranges from 50ng to 150ng/ml, since it was first measured in chicken brain [37]. Winlson and his colleagues have found a maximal activity on angiogenesis when netrin-1’s concentration at 50ng/ml [38]. Otherwise, there was no significant increase in endothelial cell migration for netrin-1 concentration superior to 1 µg/ml. It indicated that high doses netrin-1 could lose its ability of inducing endothelial cells migration and even prevents endothelial cells movements. Moreover dose-reponse effect has already been observed for other angiogenic molecules such as endostatin, thrombospondin-1, angiopoietin and even for PDGF, FGF and VEGF on other cell types, such as mast cells for example [39-41]. Thus, physical concentration of netrin-1 can measure in normal and pathological human tissue, and whether the angiogenesis in retina of DR patient is due to decline dose of netrin-1 to 50ng/ml.
We have known that UNC5B is a dependence receptor. Regulation of endothelial cell apoptosis may be another mechanism to finely tune angiogenesis. As unc5b can induce a survival signal when bonding with UNC5B, stimulate proliferation, and stabilize the existed vessel. What’s more, UNC5B can transmit a molecular cascade leading to cell death without netrin-1. Induction of apoptosis by increasing unc5b expression leads to sprouting of new capillaries and more filopodias, whereas promotion of endothelial cell survival can make vessels quiescence or stable. Retinal endothelial tip cells express high levels of unc-5 homolog b (UNC5b). Lu X et al have found that compared to uninjected control eyes, netrin-1-injected eyes showed a marked decrease in filopodial extension, while filopodial retraction induced by netrin-1 was completely neutralized by preincubation of netrin-1 protein with recombinant UNC5B–Fc [27]. Knockout of the Unc5b gene in mice which prevent the survival signal from stimulated by netrin-1 leads to aberrant extension of endothelial tip cell filopodia, excessive vessel branching and abnormal navigation. Overall angiogenesis may come along after the signal of apoptosis is evoked. What’s more ,it has been proved that in the developing avian embryos [33]. But it still needs further studies and more molecular evidences to prove this point.
However, Nguyen and Cai [42] demonstrated another mechanism of netrin-1 induces angiogenesis. NO which promotes endothelial cell survival by inhibiting the cysteine protease activity of caspases via S-nitrosylation of the reactive cysteine residue [43]. In fact, induction of angiogenesis by netrin-1 is NO-dependent and stimulation of NO requires extracellular signal-regulated kinase (ERK)1/2 and DCC . Netrin-1 activates DCC resulting in activation of ERK1/2 and subsequently endothelial NO production from serine phosphorylated eNOS. NO also contributes to ERK1/2 activation forming a feed-forward cycle. This is another evidence to prove DCC can stimulate angiogenesis. However, except inhibitor apoptosis signal activating, NO can also cause hypoxic-metabolic through competing hemoglobin and cytochrome-c oxidase with oxygen, regeneration of mitochondrion and angiogenesis. Consequently, NO mediates netrin-1-induced enhancement in endothelial cell growth and migration. And the effect of netrin-1 on angiogenesis can be supposed in this way.
Apart from above, recent study shows that netrin-1 can activate of RhoA, cathepsin B(CatB), and cAMP-response element-binding protein(CREB) to promote angiogenesis [44]. Inhibiting the specific CatB and silencing CREB can vessel sprouting induced by netrin-1. However a study demonstrated that netrin-4 expression by muller cell and retinal pigment epithelium cells can increase phosphorylation of MAPK, ERK1/2 and p38 ,and control retinal angiogenesis [45]. Another study in zebrafish development underscores the crucial role of Netrin-4 in blood vessel formation and the involvement of protein kinases activation by Netrin-4-induced biological effects [30]. These findings suggest that netrin-1/4 can serve as an innovative agent for the treatment in angiogenesis related diseases [46]. Likewise, a study in colorectal cancer carcinoma shows that netrin-4 is implicated in angiogenesis in tumor angiogenesis, making it vital to the survival of the cancer patient [47]. The discovery of this pathway offers an important perspective for therapeutic angiogenesis in patients with ischemic diseases such as hind limb ischemia [48], placental vasculature-related diseases [49], and diabetic retinopathy [50].
Netrin-1 in Diabetic Retinopathy
Angiogenesis is the underlying cause of more than 70 disorders [51] It is also crucial for PDR. Abnormal retinal vessel growth is initiated by the hypoxia and inflammation, enhanced by vascular endothelial dysfunction, which triggers an vascular permeability [52]. Unlike other ischemic complication caused by diabetes, diabetic retinopathy is characteristic by angiogenesis in normal avascular area. Retina is hypermetabolism tissue which needs more energy and the blood to take surplus oxyradical and production of metabolism. While the high blood sugar and hypoxia can’t provide sufficient ATP to meet demand, it comes to vascular endothelial dysfunction and vascular permeability, then more abnormal angiogenesis for compensation. As retina belong to part of central nerves system, and netrin-1’s dual effect on angiogenesis. Thus, inhibiting angiogenesis by targeting netrin-1 represent a promising avenue for preventing diabetic retinopathy process.
Infiltration of neutrophils and monocytes is one of the main causes leading to angiogenesis in PDR. Cytokines, such as IL-17 and IFN-γ, produced by neutrophils are known to mediate endothelial cell and pericyte cell injury. And studies have proved that hypoxia can induce netrin-1 through hypoxia-inducible factor (HIF), and Netrin-1 regulates inflammation through NFκB and COX-2/PGE2 pathways. And UNC5B receptor mediates netrin-1 anti-inflammatory effects [8,53]. Other evidence was demonstrated that Netrin-1 could reverse neovascularization and suppressed inflammation in alkali-burned cornea [54]. So in this level, we can think netrin-1 is a protect factor in DR. We can speculate that netrin-1 secreted by retina is decreasing as neuropathy in diabetes, and the anti-inflammation and suppression effect that cause angiogenesis in retina has been weaken.
However, it has been shown that netrin-1 may act as pro-angiogenesis factor and promote the progress of retinopathy [55]. Indeed, some studies have showed that netrin-1 is an pro-angiogenic factor with the unique ability to attract both blood vessels and axons [28,29,31]. Studies show that netrin-1 is abundantly expressed in vitreous of PDR patients [56]. What’s more, Netrin-1 significantly increases retinal revascularization in oxygen-induced retinopathy (OIR) model [57]. Afterward a study demonstrats that using small hairpin RNA(shRNA) to degration netrin-1 is an effective method to reduce pathological angiogenesis in retina [50]. So targeting netrin-1 might be attractive as a treatment for diabetic retinopathy, either by inhibiting unc5b expression or by preventing its downstream signal receptors.
Discussion
In addition to its role in the nervous system during embryogenesis, netrin-1 also plays an important role in other systems such as oncology, cardiology and ophthalmology. It has become apparently in the last several years that guidance molecules are involved in retinal angiogenesis development through interacting with cell survival, migration and angiogenic pathways. In the field of ophthalmology, netrin-1 is involved in ocular angiogenesis which promotes vitreous hemorrhage. All these data emphasize that the guidance molecules, Netrin-1/DCC/UNC5, provide another way to treat DR. On a cautionary note, there is considerable complexity in these pathways. For example, netrin-1 and their receptors, in the endothelium, are more complex that signaling may not mediate through the unique endothelium receptor, Unc5B. Identification of new binding partners as well as new receptors for netrin-1 receptors, have reinforced this hypothesis. Moreover, there appears to be cross-talk between other guidance molecules like EPH/ephrin, Slit/Robo, semaphorins/Neuropilins/plexins and Delta/JAG/Notch pathways. Nevertheless, this represents an exciting area of investigation in diabetic retinopathy and promise for the future treatment.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81371036)
References
-
Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G (2012) Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol 181: 1991-2002.
-
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123-1133.
-
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436: 193-200.
-
Gelfand MV, Hong S, Gu C (2009) Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol 19: 99-110.
-
Stem MS, Gardner TW (2013) Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications. Curr Med Chem 20: 3241-3250.
-
Simó R, Hernández C; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) (2012) Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br J Ophthalmol 96: 1285-1290.
-
Nikolopoulos SN, Giancotti FG (2005) Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle 4: e131-135.
-
Ranganathan P, Mohamed R, Jayakumar C, Ramesh G2 (2014) Guidance cue netrin-1 and the regulation of inflammation in acute and chronic kidney disease. Mediators Inflamm 2014: 525891.
-
Arakawa H (2004) Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer 4: 978-987.
-
Bagri A, Ashkenazi A (2010) UNCovering the molecular machinery of dependence receptor signaling. Mol Cell 40: 851-853.
-
Cirulli V, Yebra M (2007) Netrins: beyond the brain. Nat Rev Mol Cell Biol 8: 296-306.
-
Schön MP, Schön M (2009) To die or not to die, that's the question--and the answer may depend on netrin-1. J Natl Cancer Inst 101: 217-219.
-
Mille F, Llambi F, Guix C, Delloye-Bourgeois C, Guenebeaud C, et al. (2009) Interfering with multimerization of netrin-1 receptors triggers tumor cell death. Cell Death Differ 16: 1344-1351.
-
Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A (2009) Guidance of vascular development lessons from the nervous system. Circ Res 104: 428-441.
-
Baioni L, Basini G, Bussolati S, Cortimiglia C, Grasselli F (2010) Netrin-1: just an axon-guidance factor? Vet Res Commun 34 Suppl 1: S1-4.
-
Manitt C, Nikolakopoulou AM, Almario DR, Nguyen SA, Cohen-Cory S (2009) Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci 29: 11065-11077.
-
Shirkey NJ, Manitt C, Zuniga L, Cohen-Cory S (2012) Dynamic responses of Xenopus retinal ganglion cell axon growth cones to netrin-1 as they innervate their in vivo target. Dev Neurobiol 72: 628-648.
-
Johansson K, Törngren M, Wasselius J, Månsson L, Ehinger B (2001) Developmental expression of DCC in the rat retina. Brain Res Dev Brain Res 130: 133-138.
-
Shi M, Zheng MH, Liu ZR, Hu ZL, Huang Y, et al. (2010) DCC is specifically required for the survival of retinal ganglion and displaced amacrine cells in the developing mouse retina. Dev Biol 348: 87-96.
-
Soto F, Watkins KL, Johnson RE, Schottler F, Kerschensteiner D (2013) NGL-2 regulates pathway-specific neurite growth and lamination, synapse formation, and signal transmission in the retina. J Neurosci 33: 11949-11959.
-
Brunet I, Gordon E, Han J, Cristofaro B, Broqueres-You D, et al. (2014) Netrin-1 controls sympathetic arterial innervation. J Clin Iinvest 124: 3230-3240.
-
Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C (2011) Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev 25: 1399-1411.
-
Dorrell MI, Friedlander M (2006) Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res 25: 277-295.
-
Tian XF, Xia XB, Xiong SQ, Jiang J, Liu D, et al. (2011) Netrin-1 overexpression in oxygen-induced retinopathy correlates with breakdown of the blood-retina barrier and retinal neovascularization. Ophthalmologica 226: 37-44.
-
Mancino M, Ametller E, Gascón P, Almendro V (2011) The neuronal influence on tumor progression. Biochim Biophys Acta 1816: 105-118.
-
Larrivée B, Freitas C, Trombe M, Lv X, Delafarge B, et al. (2007) Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev 21: 2433-2447.
-
Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, et al. (2004) The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432: 179-186.
-
Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, et al. (2008) The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development 135: 659-667.
-
Castets M, Coissieux MM, Delloye-Bourgeois C, Bernard L, Delcros JG, et al. (2009) Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev Cell 16: 614-620.
-
Lambert E, Coissieux MM, Laudet V, Mehlen P (2012) Netrin-4 acts as a pro-angiogenic factor during zebrafish development. J Biol Chem 287: 3987-3999.
-
Castets M, Mehlen P (2010) Netrin-1 role in angiogenesis: to be or not to be a pro-angiogenic factor? Cell Cycle 9: 1466-1471.
-
Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G (2013) Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int 83: 1087-1098.
-
Bouvrée K, Larrivée B, Lv X, Yuan L, DeLafarge B, et al. (2008) Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol 318: 172-183.
-
Adams RH, Eichmann A (2010) Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2: a001875.
-
Xie H, Zou L, Zhu J, Yang Y (2011) Effects of netrin-1 and netrin-1 knockdown on human umbilical vein endothelial cells and angiogenesis of rat placenta. Placenta 32: 546-553.
-
Cayre M, Courtès S, Martineau F, Giordano M, Arnaud K, et al. (2013) Netrin 1 contributes to vascular remodeling in the subventricular zone and promotes progenitor emigration after demyelination. Development 140: 3107-3117.
-
Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, et al. (1996) Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87: 1001-1014.
-
Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, et al. (2004) The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A 101: 16210-16215.
-
Motegi K, Harada K, Pazouki S, Baillie R, Schor AM (2002) Evidence of a bi-phasic effect of thrombospondin-1 on angiogenesis. Histochem J 34: 411-421.
-
Fu Y, Tang H, Huang Y, Song N, Luo Y (2009) Unraveling the mysteries of endostatin. IUBMB Life 61: 613-626.
-
Fagiani E, Christofori G (2013) Angiopoietins in angiogenesis. Cancer Lett 328: 18-26.
-
Nguyen A, Cai H (2006) Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A 103: 6530-6535.
-
Dimmeler S, Zeiher AM (2000) Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res 87: 434-439.
-
Shimizu A, Nakayama H, Wang P, König C, Akino T, et al. (2013) Netrin-1 Promotes Glioblastoma Cell Invasiveness and Angiogenesis by Multiple Pathways Including Activation of RhoA, Cathepsin B, and cAMP-response Element-binding Protein. J Biol Chem 288: 2210-2222.
-
Lange J, Yafai Y, Noack A, Yang XM, Munk AB, et al. (2012) The axon guidance molecule Netrin-4 is expressed by Müller cells and contributes to angiogenesis in the retina. Glia 60: 1567-1578.
-
Ko SY, Blatch GL, Dass CR (2014) Netrin-1 as a potential target for metastatic cancer: focus on colorectal cancer. Cancer Metastasis Rev 33: 101-113.
-
Eveno C, Broqueres-You D, Feron JG, Rampanou A, Tijeras-Raballand A, et al. (2011) Netrin-4 delays colorectal cancer carcinomatosis by inhibiting tumor angiogenesis. Am J Pathol 178: 1861-1869.
-
Li Q, Yao D, Ma J, Zhu J, Xu X, et al. (2011) Transplantation of MSCs in combination with netrin-1 improves neoangiogenesis in a rat model of hind limb ischemia. J Surg Res 166: 162-169.
-
Wang Q, Zhu J, Zou L, Yang Y (2011) Role of axonal guidance factor netrin-1 in human placental vascular growth. J Huazhong Univ Sci Technolog Med Sci 31: 246-250.
-
Xu H, Liu J, Xiong S, Le YZ, Xia X (2012) Suppression of retinal neovascularization by lentivirus-mediated netrin-1 small hairpin RNA. Ophthalmic Res 47: 163-169.
-
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438: 932-936.
-
Kastelan S, Tomic M, Gverovic Antunica A, Salopek Rabatic J, Ljubic S (2013) Inflammation and pharmacological treatment in diabetic retinopathy. Mediators Inflamm 2013: 213130.
-
Eltzschig HK (2009) Adenosine: an old drug newly discovered. Anesthesiology 111: 904-915.
-
Han Y, Shao Y, Lin Z, Qu YL, Wang H, et al. (2012) Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci 53: 1285-1295.
-
Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, et al. (2004) The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A 101: 16210-16215.
-
Liu J, Xia X, Xiong S, Le Y, Xu H (2011) Intravitreous high expression level of netrin-1 in patients with proliferative diabetic retinopathy. Eye Sci 26: 85-90, 120.
-
Binet F, Mawambo-Tagne GS, Favret S, Sitaras N, Tétreault N, et al. (2012) Netrin-1 Promotes Vascular Regeneration in a Mouse Model of Ischemic Retinopathy. Investigative Ophthalmology & Visual Science 53 (E-Abstract): 6298.
-
Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, et al. (1994) The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78: 409-424.
-
de la Torre JR, Höpker VH, Ming GL, Poo MM, Tessier-Lavigne M, et al. (1997) Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron 19: 1211-1224.
-
Lauderdale JD, Davis NM, Kuwada JY (1997) Axon tracts correlate with netrin-1a expression in the zebrafish embryo. Mol Cell Neurosci 9: 293-313.
-
Meyerhardt JA, Caca K, Eckstrand BC, Hu G, Lengauer C, et al. (1999) Netrin-1: interaction with deleted in colorectal cancer (DCC) and alterations in brain tumors and neuroblastomas. Cell Growth Differ 10: 35-42.
-
Van Raay TJ, Foskett SM, Connors TD, Klinger KW, Landes GM, et al. (1997) The NTN2L gene encoding a novel human netrin maps to the autosomal dominant polycystic kidney disease region on chromosome 16p13.3. Genomics 41: 279-282.
-
Wang H, Copeland NG, Gilbert DJ, Jenkins NA, Tessier-Lavigne M (1999) Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J Neurosci 19: 4938-4947.
-
Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, et al. (2000) A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol 151: 221-234.
-
Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, et al. (1999) A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97: 927-941.
-
Huber AB, Kolodkin AL, Ginty DD, Cloutier JF (2003) Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci 26: 509-563.
-
Xu Z, Li H, Wadsworth WG (2009) The roles of multiple UNC-40 (DCC) receptor-mediated signals in determining neuronal asymmetry induced by the UNC-6 (netrin) ligand. Genetics 183: 941-949.
-
Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, et al. (1992) UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71: 289-299.
-
Keleman K, Dickson BJ (2001) Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32: 605-617.





