International Journal of Cancer and Clinical Research
Metastatic Pancreatic Adenocarcinoma to a Lymph Node with Diffuse Large B Cell Lymphoma: A Case Report and Review of the Literature
Xiao Huang2, Kazunori Kanehira1, Fadi Habib1 and Bo Xu1*
1Department of Pathology and Laboratory Medicine, Roswell Park Cancer Institute, Buffalo, NY, USA
2Department of Pathology, George Washington University Hospital, Washington, DC, USA
*Corresponding author:
Bo Xu, Department of Pathology and Laboratory Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA, Tel: 716-845-5981, E-mail: bo.xu@roswellpark.org
Int J Cancer Clin Res, IJCCR-3-045, (Volume 3, Issue 1), Case Report; ISSN: 2378-3419
Received: January 07, 2016 | Accepted: February 16, 2016 | Published: February 19, 2016
Citation: Huang X, Kanehira K, Habib F, Xu B (2016) Metastatic Pancreatic Adenocarcinoma to a Lymph Node with Diffuse Large B Cell Lymphoma: A Case Report and Review of the Literature. Int J Cancer Clin Res 3:045. 10.23937/2378-3419/3/1/1045
Copyright: © 2016 Huang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Metastatic carcinoma to a lymph node with high grade lymphoma is very rare, particularly when high grade lymphoma is the first time diagnosis. Here we report a case where metastatic pancreatic ductal adenocarcinoma was found in a retroperitoneal lymph node with diffuse large B cell lymphoma (DLBCL). Histologically, the metastatic adenocarcinoma is located mainly in the subcapsular region. The lymph node architecture is diffusely effaced by proliferation of medium to large atypical lymphoid cells with prominent nucleoli, eosinophilic cytoplasm and eccentric nuclei. Immunohistochemical studies revealed these neoplastic lymphocytes are consistent with diffuse large B-cell lymphoma with immunoblastic morphology. Literatures on concomitant metastatic carcinoma with lymphoma in the same lymph node were reviewed. The significance and underline biological mechanisms were also discussed.
Keywords
Concomitant metastatic pancreatic ductal adenocarcinoma, Lymph node, Diffuse large B cell lymphoma
Introduction
The occurrence of multiple primary malignant tumors in the same individual is well reported [1,2], whereas coexistence of two malignancies in the same organ/site is uncommon [3,4]. The second malignancy may be unexpectedly discovered during the investigation for previous malignant tumor [5]. It has been reported previously that patients with small cell lymphoma/chronic lymphocytic leukemia (SLL/CLL), have an increased risk of developing other malignancies, particularly squamous-cell carcinoma [6-8]. However, metastasis of adenocarcinoma to a lymph node harboring diffuse large B cell lymphoma (DLBCL) is extremely rare. Here we report a case that DLBCL was unexpectedly discovered in a patient’s retroperitoneal lymph node with metastatic moderately differentiated pancreatic ductal adenocarcinoma.
Case Presentation
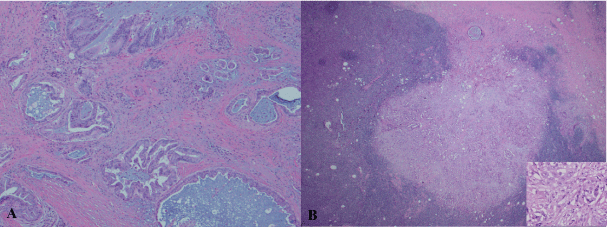
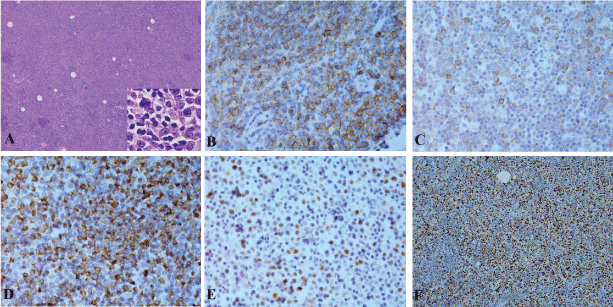
A 79-year-old Caucasian male presented with chest pruritus, darkening urine and clay-colored stools for 2 months. This diabetic patient has family history of pancreatic cancer (father). Accompanying symptoms included depression, dizziness, dry mouth and recent weight loss of 6.8 kg. There was no fever or night sweats. Computer tomography showed a 1.9 cm mass in the head of pancreas, and several less than 1 cm lymph nodes in the peripancreatic aortocaval celiac. No significant adenopathy was identified in chest and pelvis. Magnetic resonance cholangiopancreatography (MRCP) showed dilatation of both the common bile duct and the pancreatic duct, suggesting a lesion of pancreatic head. Endoscopic ultrasound (EUS) guided fine-needle aspiration (FNA) biopsies of the pancreatic mass revealed adenocarcinoma. Pancreaticoduodenectomy was performed with feeding jejunostomy. During the surgery, a bulky lymph node, 2.5 cm in size was identified, which is directly behind the C-loop of the duodenum and is within 1 cm to common bile duct margin. This lymph node was mobilized and dissection of regional lymph nodes was performed. Grossly, within the pancreatic head, there is an ill defined tan to white mass, 4 × 3 × 3 cm adjacent to common bile duct and duodenum. Tumor is confined to the pancreatic parenchyma. Histological examination reveals an infiltrating moderately differentiated pancreatic ductal adenocarcinoma (Figure 1). Examination of regional lymph nodes shows metastatic adenocarcinoma in 8 out of 18 lymph nodes, and among the 8 positive lymph nodes, one also displays features of diffuse large B cell lymphoma. As illustrated in figure 2, metastatic adenocarcinoma was seen predominantly in the subcapsular area (Figure 1). In addition, the architecture of the lymph node is diffusedly effaced by proliferation of medium to large atypical lymphoid cells with prominent nucleoli, eosinophilic cytoplasm and some with eccentric nuclei. Small reactive lymphocytes are seen in the background. Few residual follicles are also noted (Figure 2). The neoplastic lymphocytes are positive for CD45, CD20, Pax-5, CD79a, Bcl-2, MUM-1, CD43 (Figure 2), and negative for CD10, Bcl-6, CD30, CD3, CD4, CD7, CD8, TIA, Granzyme-B, CD138, kappa, lambda and EBV-LMP. CD5 immunostain shows focal weak staining of neoplastic lymphoid cells. CD23 and CD21 highlight the residual follicular dendritic cell meshwork. The MIB-1 proliferation index is approximately 60-70%. The overall morphologic features and immunostain profiles are consistent with DLBCL with immunoblastic morphology. Pathological stage of pT2N1M0 for pancreatic adenocarcinoma was assigned with clinical stage of IIB. The anatomic stage/prognostic group for DLBCL is stage I. The patient was given adjuvant chemotherapy for pancreatic adenocarcinoma with gemcitabine, 5-FU and leucovorin. After 10 cycles of chemotherapy, peritoneal recurrence of metastatic adenocarcinoma was identified. Although Leucovorin and 5-FU were added in the treatment regiment, progress of disease was not controlled. Fifteen months after Whipple procedure, the patient was transferred to hospice care. No treatment was given for DLBCL post surgery.
.
Figure 1: Pancreatic ductal adenocarcinoma (A) and its metastasis to a retroperitoneal lymph node (B). H & E stain, A: 40 x and B: 40 x with insert 200 x.
View Figure 1
.
Figure 2: DLBCL of a lymph node with metastatic pancreatic adenocarcinoma. A: H&E stain, 40 x with insert 200 x, B-F: Immunostains for CD20 (B), CD79a (C), BCL-2 (D), MUM-1 (E) and Ki67 (F). B-E, 200 x, F, 40 x.
View Figure 2
Discussion
Collision tumors are rare entities defined by presence of two neoplasms of distinct origin found in a single anatomic location [9,10]. The mechanism of the second primary malignancy development is unclear. There are multiple hypotheses among the pathogenic mechanisms to explain the increased risk of second tumor, such as mutagenic effects of radiation and chemotherapy, genetic predisposition, and environmental factors [6]. In our case, metastatic pancreatic ductal adenocarcinoma and DLBCL were identified in the same lymph node. DLBCL is an aggressive lymphoma and can involve the whole lymphatic system. However, the DLBCL was unexpectedly identified in a retroperitoneal lymph node and not found in other site/organ in this patient. It has been speculated that DLBCL may increase the risk for developing other non-hematological malignancies [11-13].
Pancreatic cancer is the fourth leading cause of cancer death in the United States, and it has the worst prognosis of any major tumor type. There are some risk factors of developing pancreatic cancer, such as family history [14], cigarette smoking [15], type 2 diabetes [16], increasing body mass index [17], and heavy alcohol consumption [18]. There is also 20-40% higher incidence of malignancy, such as pancreatic carcinoma and non-Hodgkin’s lymphoma, in type 2 diabetes patients [19]. In our case, patient's family history of pancreatic cancer and his diabetes may be contributing factors to the development of pancreatic carcinoma. It is not clear whether the microenvironment created by the first malignancy in lymph node potentiates development of the second malignancy. More studies need to be done to further our understanding of the mechanisms for this rare collision of malignant tumors.
References
-
Warren S, Gates O (1932) Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer 16: 1358-1414.
-
Watson TA (1953) Incidence of multiple cancer. Cancer 6: 365-371.
-
Rabson Sm, Stier P, Baumgartner Jc, Rosenbaum D (1954) Metastasis of cancer to cancer. Am J Clin Pathol 24: 572-579.
-
Allal AS, Weintraub J, Remadi S, Abele R (1996) Concurrent interfollicular Hodgkin's disease and metastatic breast carcinoma in lymph nodes. Pathol Int 46: 787-790.
-
Pandey U, Naraynan M, Karnik U, Sinha B (2003) Carcinoma metastasis to unexpected synchronous lymphoproliferative disorder: report of three cases and review of literature. J Clin Pathol 56: 970-971.
-
Caraway NP, Wojcik EM, Saboorian HM, Katz RL (1997) Concomitant lymphoma and metastatic carcinoma in a lymph node: diagnosis by fine-needle aspiration biopsy in two cases. Diagn Cytopathol 17: 287-291.
-
Perez-Reyes N, Farhi DC (1987) Squamous cell carcinoma of head and neck in patients with well-differentiated lymphocytic lymphoma. Cancer 59: 540-544.
-
Frierson HF Jr, Deutsch BD, Levine PA (1988) Clinicopathologic features of cutaneous squamous cell carcinomas of the head and neck in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum Pathol 19: 1397-1402.
-
Kakarala K, Sadow PM, Emerick KS (2010) Cervical lymph node collision tumor consisting of metastatic squamous cell carcinoma and B-cell lymphoma. Laryngoscope 120: S156.
-
Dong C, Hemminki K (2001) Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: a search for common mechanisms. Br J Cancer 85: 997-1005.
-
Hu XR, Hu YX, Fu HR, Sheng LX, Huang WJ, et al. (2011) Diffuse large B-cell lymphoma with concurrent gastric adenocarcinoma: case report and literature review. J Int Med Res 39: 2051-2058.
-
Yeh YM, Chang KC, Chen YP, Kao LY, Tsai HP, et al. (2010) Large B cell lymphoma presenting initially in bone marrow, liver and spleen: an aggressive entity associated frequently with haemophagocytic syndrome. Histopathology 57: 785-795.
-
Morton LM, Curtis RE, Linet MS, Bluhm EC, Tucker MA, et al. (2010) Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 28: 4935-4944.
-
Klein AP (2013) Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer 13: 66-74.
-
Iodice S, Gandini S, Maisonneuve P, Lowenfels AB (2008) Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 393: 535-545.
-
Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, et al. (2005) Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129: 504-511.
-
Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, et al. (2010) Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 170: 791-802.
-
Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, et al. (2012) Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 23: 374-382.
-
Rendell M, Akturk HK, Tella SH (2013) Glargine safety, diabetes and cancer. Expert Opin Drug Saf 12: 247-263.