International Journal of Cancer and Clinical Research
Incidental Paget's Disease of the Nipple in a Prophylactic Skin-Sparing Mastectomy for BRCA1 Mutation: Implications for Increased Use of Prophylactic Nipple Sparing Mastectomy
Esther Dubrovsky1, Farayan Jalalabi2, Aysegul Sahin3 and Dalliah M Black4*
1Department of Surgery, Houston Methodist Hospital, Houston, USA
2University of Texas Health Sciences Center at Houston, USA
3Department of Pathology, The University of Texas, MD Anderson Cancer Center, Houston, USA
4Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, USA
*Corresponding author:
Dalliah M Black, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1434, Houston, Texas 77030, Tel: 713-792-4236, Fax: 713-794-5720, E-mail: dmblack@mdanderson.org
Int J Cancer Clin Res, IJCCR-3-044, (Volume 3, Issue 1), Case Report; ISSN: 2378-3419
Received: October 31, 2015 | Accepted: February 08, 2016 | Published: February 10, 2016
Citation: Dubrovsky E, Jalalabi F, Sahin A, Black DM (2016) Incidental Paget's Disease of the Nipple in a Prophylactic Skin-Sparing Mastectomy for BRCA1 Mutation: Implications for Increased Use of Prophylactic Nipple Sparing Mastectomy. Int J Cancer Clin Res 3:044. 10.23937/2378-3419/3/1/1044
Copyright: © 2016 Dubrovsky E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Prophylactic skin-sparing mastectomy (SSM) and nipple sparing mastectomy (NSM) have become suitable options for breast cancer prevention in appropriate patients with hereditary risk of breast cancer [1]. Prophylactic total mastectomy (TM) provides approximately 90% to 95%- risk reduction in patients with BRCA1 and BRCA 2 mutations [2]. Initially, bilateral TM was recommended because of the concern for an increased breast cancer risk from remaining residual breast tissue. However, studies have demonstrated that SSM and NSM are feasible options for prophylactic surgery in patients with BRCA mutations and provide the opportunity for reconstruction with excellent outcomes [3-5]. We report a case in which a woman with a BRCA1 mutation underwent prophylactic bilateral SSM and salpingo-Oophorectomy with Paget's disease incidentally found in the left nipple on pathology evaluation. Incidental Paget's disease in prophylactic mastectomy specimens raises the concern for continued long term follow-up of high risk patients undergoing NSM.
Keywords
Paget's disease, Prophylactic mastectomy, BRCA mutation
Introduction
Paget's disease of the nipple is an uncommon presentation of breast cancer and usually presents with nipple excoriation or ulceration with or without an underlying invasive cancer, but it can be found incidentally on mastectomy specimens performed for invasive cancer [6-8]. We report a case of a 35 year old woman with a BRCA 1 mutation who was found to have incidental Paget's disease of the nipple without an underlying invasive or in situ carcinoma component from a prophylactic skin-sparing mastectomy.
Case Report
A 35 year old premenopausal female with a family history of breast cancer and a known BRCA1 mutation presented to discuss surgical options for breast cancer risk reduction. Her family history was significant for two paternal aunts with breast cancer at ages 40 and 59, and one cousin with breast cancer at age 22 with a recurrence at age 30. Her older paternal aunt and cousin tested positive for a BRCA1 mutation (1793delA). Given her initial desire to have children and breastfeed, the patient elected to undergo high-risk screening, alternating bilateral mammogram and breast MRI every 6 months. Imaging studies and physical exam were negative for abnormalities. After one and a half years of high risk screening, she sought counsel regarding surgical prophylaxis.
The patient's oncologic history was notable for papillary thyroid cancer (T1N1a), for which she underwent total thyroidectomy, bilateral paratracheal and superior mediastinal node dissection, and auto transplantation of the left inferior parathyroid into the left trapezius muscle. She declined radioactive iodine therapy because of her desire to conceive. Her past medical history was otherwise negative. She was referred for genetic counseling after the thyroid cancer diagnosis and tested positive for a familial BRCA1 mutation (1793delA). The patient then learned of her cousin's BRCA1 mutation. The geneticist estimated the patient's lifetime risk for bilateral breast cancer at approximately 50% to 80% by age 70 years and risk for ovarian cancer up to 45% by age 70 years.
At an elective surgical consultation, prophylactic bilateral skin sparing mastectomy, nipple sparing mastectomy, and total mastectomy was discussed and compared to continued high risk screening with or without salpingo-oophorectomy which would provide an approximately 50% breast cancer risk reduction. The most recent bilateral digital mammogram and breast MRI with 1.5 Tesla showed no abnormalities.
Physical exam revealed no abnormal lymphadenopathy, skin changes or skin dimpling, no nipple changes or excoriation or discharge, and no palpable breast masses. The patient was not an ideal candidate for nipple sparing mastectomy given grade II ptosis. After consultation with plastic surgery, the patient elected to have bilateral skin-sparing mastectomy with immediate tissue expander reconstruction combined with bilateral salpingo-oophorectomy by the gynecologist. The patient did well after surgery with no complications and had final reconstruction with bilateral silicone implants.
Pathology
On pathologic evaluation, both breasts showed no evidence of invasive or in situ cancer or any high risk proliferative lesions. However, the left nipple showed focal atypical cells in the nipple. The differential included toker cells, an atypical melanocytic proliferation, and Paget's disease. The left breast was extensively sampled, revealing no underlying malignancy. Immunohistochemical staining was performed on sections of the nipple which showed atypical cells in the epidermis with positive staining for cytokeratin 7, estrogen and progesterone receptors, E-cadherin and Ki-67. The cells were negative for S100 protein, Her-2/neu over-expression, and CEA. (Figure 1 and Figure 2)
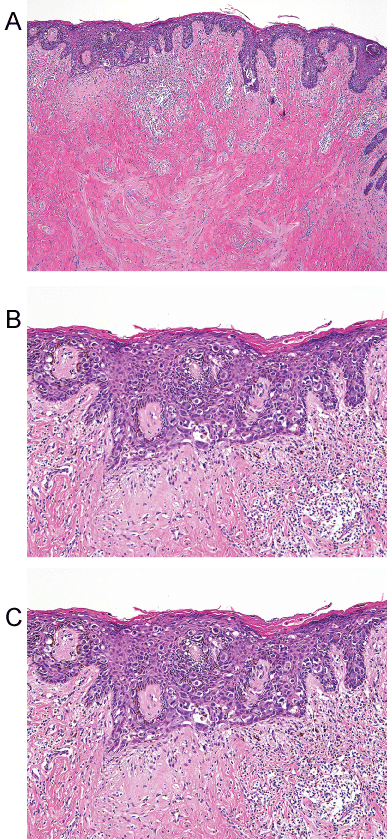
.
Figure 1: Hematoxylin and Eosin sections of the nipple. (A) Characteristic carcinoma cells (Paget's cells) form clusters in the deep epidermis and they are scattered individually throughout the epidermis which shows focal hyperkeratosis. Note the dermal lymphocytic infiltrate which is a frequent finding; (B) The Paget's cells are characterized by pale cytoplasm; (C) The nuclei are large and pleomorphic. Prominent nuclei are evident.
View Figure 1
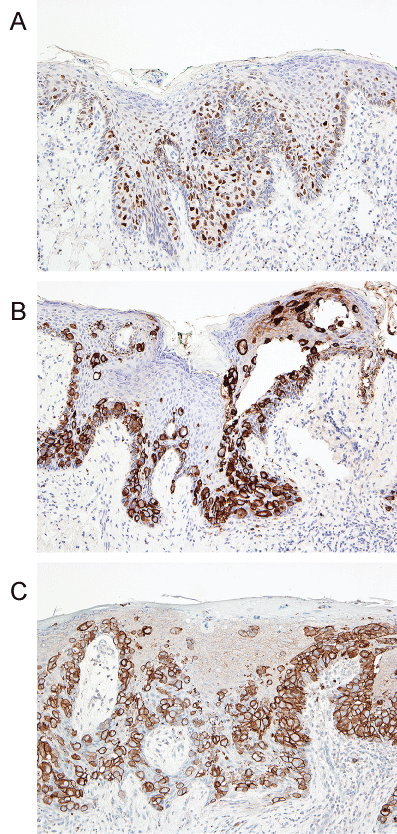
.
Figure 2: Immunohistochemical staining. (A) GATA-3 staining highlights Paget's cells. Note negative epidermal cells in the background; (B) Keratin-7 is positive in Paget's cells and negative in epidermal keratinocytes; (C) Membrane staining for Her2/neu protein highlights Paget's cells.
View Figure 2
Discussion
To our knowledge, this case is unique in finding incidental Paget's disease of the nipple on a prophylactic skin sparing mastectomy for BRCA mutation. Increased genetic testing in unaffected women who pursue prophylactic mastectomy may lead to more detection of incidental malignancies even in the era of breast MRI screening. Patients undergoing nipple sparing mastectomy who also have increased risk of breast cancer should be aware of the chance, albeit small, of future malignancy involving the nipple.
Several studies report a low incidence of nipple related abnormalities during prophylactic mastectomy. Eisenberg et al. reviewed 117 patients who underwent prophylactic NSM and showed no histologic abnormality or malignancy in any of the nipple margins [9]. Other series have examined the histologic findings in mastectomy specimens from patients with hereditary risk factors or BRCA mutations. One series showed an odds ratio of 12.7 for the detection of any high-risk lesion (ductal carcinoma in situ, lobular carcinoma in situ, atypical ductal hyperplasia, or atypical lobular hyperplasia) in specimens from BRCA mutation carriers [10]. Another series of 67 women found 57% had an atypical finding within the prophylactic mastectomy specimen: 37% atypical lobular hyperplasia, 39% atypical ductal hyperplasia, 25% lobular carcinoma-in-situ, and 15% ductal carcinoma-in-situ. The presence of high-risk lesions was independently related to age over 40 years (odds ratio 6.6), and inversely related to bilateral oophorectomy before prophylactic mastectomy (odds ratio 0.2) [11]. None of these series reported any significant findings within the nipple. Patients with a clinically negative exam and no detectable malignancy in the mastectomy specimen have an extremely low likelihood of having significant disease within the nipple.
Paget's disease refers to a clinical diagnosis characterized by a scaly, ulcerated, and raw appearance to the nipple. Paget's disease of the breast was first described by Sir James Paget in 1874, when he published his findings of 15 women with ulceration of the nipple followed by cancer of the mammary gland. The overall incidence of Paget's disease is low among women with breast disease, ranging from 1-4.5% [12,13]. The majority of patients (85-88%) with Paget's disease of the nipple have an underlying invasive malignancy, and in 25% of cases there is no clinical or radiographic evidence of the occult tumor [14-17]. However, most of these studies were done prior to routine use of breast MRI screening.
Historically, Paget's disease is thought to be an epidermotropic carcinoma. In most cases, there is an invasive or in situ carcinoma in the underlying breast parenchyma and tumor cells originating in breast ducts migrate to nipple epidermis either by through duct system or direct invasion. In the majority of cases an underlying carcinoma is identified. However, there are rare Paget's disease cases in which no underlying carcinoma is identified despite extensive sampling [18]. The characteristic histopathologic feature of Paget's disease is the presence of ductal carcinoma cells (Paget cells) within the keratinizing epithelium of the nipple epidermis. Paget's cells may occur single or form clusters. Clustering of Paget's cells is usually more common in the basal portion of epidermis. Paget's cells rarely form glands within the epidermis. Typically Paget's cells are large and atypical in appearance and they stand out easily from surrounding keratinocytes based on their cytological features. They have pleomorphic nuclei, often-prominent nucleoli, and abundant pale or eosinophilic nucleoli. Cytoplasm may contain mucin. Hyperplasia or hyperkeratosis of the epidermis occurs to some degree in many cases. The superficial dermis usually has mild to moderate lymphocytic infiltrate. Paget's cells have similar histochemical and immunohistochemical features with ductal carcinomas of breast. They typically are positive for cytokeratin 7, epithelial membrane antigen, and human milk fat globule. Approximately half of the cases show positivity for GCDFP-15. CEA is positive in more than 75% of Paget's cases. Her2/neu oncoprotein has been detected in 80-100% of cases [19-21].
Histologic differential diagnosis of Paget's disease includes non-neoplastic conditions of keratinocytes such as inflammatory reactions of epidermis, Toker cells, melanocytic lesions and basal cell carcinoma. Most inflammatory reactions have specific histopathologic features that are helpful to differentiate then from Paget's cells. When there is a question, immunohistohemical staining for keratin will be very helpful to identify Paget's cells. Toker cells represent a normal constituent of epidermis of nipple. They tend to localize at the tip of nipple near the opening of lactiferous sinuses. Similar to Paget's cells Toker cells have abundant clear cytoplasm but unlike Paget's cells their nuclei are usually small and lack pleomorphism. Rarely, Toker cells appear in clusters and can be confused with Paget's cells. Toker cells are positive for cytokeratin 7 but negative for epithelial membrane antigen and Her2/neu over expression. These markers can be used to distinguish Toker cells from Paget's cells. Malignant melanomas can arise in the areola but melanomas of the nipple proper are exceedingly rare. Although the histopathologic distinction might be problematic, immunohistochemical markers specific to melanoma such as HMB-45, Melan-A will help to establish the diagnosis (Table 1).
![]()
Table 1: Immunohistochemistry Features of Nipple Epidermal Lesions.
View Table 1
The treatment for clinically diagnosed Paget's disease in the absence of pre-operative evidence of underlying malignancy involves either a total mastectomy or breast conserving therapy (resection of the nipple areolar complex followed by whole-breast radiation) [7,14]. There are two pathologic theories describing the origin of Paget's disease of the breast: in situ versus epidermotropic [22]. If the origin is thought to be in situ malignant transformation, originating in the nipple epithelium, then local resection of the nipple-areolar complex followed by irradiation is sufficient treatment. However, if Paget's cells originate from ductal cancer cells that have migrated along the basal membrane of the nipple, then mastectomy is necessary. Systemic treatment is determined by the presence of an underlying malignancy.
Nipple sparing mastectomy has become an increasingly utilized technique for appropriate breast cancer patients and those at high risk for breast cancer with low incidence of future recurrences [23]. In a retrospective review of 23 BRCA positive patients who underwent prophylactic NSM, in situ cancer was incidentally found in one patient and there were no local recurrences after a mean of 51 months [22]. Another study examined the nipple areolar complex from therapeutic and prophylactic mastectomy specimens of BRCA carriers, finding no incidental disease in any of the 33 prophylactic specimens [24]. Although uncommon, the incidental pathologic finding of Paget's disease in the nipple raises the importance of continued clinical follow up in high risk patients who increasingly undergo nipple sparing mastectomy.
References
-
Sacchini V, Pinotti JA, Barros AC, Luini A, Pluchinotta A, et al. (2006) Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 203: 704-714.
-
Garwood ER, Moore D, Ewing C, Hwang ES, Alvarado M, et al. (2009) Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 249: 26-32.
-
de Alcantara Filho P, Capko D, Barry JM, Morrow M, Pusic A, et al. (2011) Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 18: 3117-3122.
-
Lanitis S, Tekkis PP, Sgourakis G, Dimopoulos N, Al Mufti R, et al. (2010) Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 251: 632-639.
-
Peled AW, Irwin CS, Hwang ES, Ewing CA, Alvarado M, et al. (2014) Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol 21: 37-41.
-
Kawase K, Dimaio DJ, Tucker SL, Buchholz TA, Ross MI, et al. (2005) Paget's disease of the breast: there is a role for breast-conserving therapy. Ann Surg Oncol 12: 391-397.
-
Caliskan M, Gatti G, Sosnovskikh I, Rotmensz N, Botteri E, et al. (2008) Paget's disease of the breast: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat 112: 513-521.
-
Marshall JK, Griffith KA, Haffty BG, Solin LJ, Vicini FA, et al. (2003) Conservative management of Paget disease of the breast with radiotherapy: 10- and 15-year results. Cancer 97: 2142-2149.
-
Eisenberg RE, Chan JS, Swistel AJ, Hoda SA (2014) Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 20: 15-21.
-
Kauff ND, Brogi E, Scheuer L, Pathak DR, Borgen PI, et al. (2003) Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer 97: 1601-1608.
-
Hoogerbrugge N, Bult P, de Widt-Levert LM, Beex LV, Kiemeney LA, et al. (2003) High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol 21: 41-45.
-
Berg JW, Hutter RV (1995) Breast cancer. Cancer 75: 257-269.
-
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61: 212-236.
-
Chen CY, Sun LM, Anderson BO (2006) Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer 107: 1448-1458.
-
Ikeda DM, Helvie MA, Frank TS, Chapel KL, Andersson IT (1993) Paget disease of the nipple: radiologic-pathologic correlation. Radiology 189: 89-94.
-
Yim JH, Wick MR, Philpott GW, Norton JA, Doherty GM (1997) Underlying pathology in mammary Paget's disease. Ann Surg Oncol 4: 287-292.
-
Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd (1998) Paget's disease of the breast: a 33-year experience. J Am Coll Surg 187: 171-177.
-
Ashikari R, Park K, Huvos AG, Urban JA (1970) Paget's disease of the breast. Cancer 26: 680-685.
-
Fu W, Lobocki CA, Silberberg BK, Chelladurai M, Young SC (2001) Molecular markers in Paget disease of the breast. J Surg Oncol 77: 171-178.
-
Sek P, Zawrocki A, Biernat W, Piekarski JH (2010) HER2 molecular subtype is a dominant subtype of mammary Paget's cells. An immunohistochemical study. Histopathology 57: 564-571.
-
Hitchcock A, Topham S, Bell J, Gullick W, Elston CW, et al. (1992) Routine diagnosis of mammary Paget's disease. A modern approach. Am J Surg Pathol 16: 58-61.
-
Karakas C (2011) Paget's disease of the breast. J Carcinog 10: 31.
-
Yao K, Liederbach E, Tang R, Lei L, Czechura T, et al. (2015) Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 22: 370-376.
-
Reynolds C, Davidson JA, Lindor NM, Glazebrook KN, Jakub JW, et al. (2011) Prophylactic and therapeutic mastectomy in BRCA mutation carriers: can the nipple be preserved? Ann Surg Oncol 18: 3102-3109.





