International Journal of Cancer and Clinical Research
Multimodal Treatment of Breast Cancer Liver Metastases Based on Hepatic Resection and Microwave Coagulo-Necrotic Therapy (MCN)
Yuko Takami1,2*, Susumu Eguchi2, Masaki Tateishi1, Tomoki Ryu1, Kazuhiro Mikagi1, Yoshiyuki Wada1 and Hideki Saitsu1
1Department of Hepato-Biliary-Pancreatic Surgery & Clinical Research Institute, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan
2Department of Surgery, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
*Corresponding author:
Yuko Takami, Department of Hepato-Biliary-Pancreatic Surgery & Clinical Research Institute, National Hospital Organization Kyushu Medical Center, 1-8-1, Jigyohama, Chuo-ku, Fukuoka 810-8563, Japan, Tel: +81-92-852-0700, Fax: +81-92-847-8802, E-mail: takabee-y@kyumed.jp
Int J Cancer Clin Res, IJCCR-3-038, (Volume 3, Issue 1), Original Article; ISSN: 2378-3419
Received: December 11, 2015 | Accepted: January 09, 2016 | Published: January 11, 2016
Citation: Takami Y, Eguchi S, Tateishi M, Ryu T, Mikagi K, et al. (2016) Multimodal Treatment of Breast Cancer Liver Metastases Based on Hepatic Resection and Microwave Coagulo-Necrotic Therapy (MCN). Int J Cancer Clin Res 3:038. 10.23937/2378-3419/3/1/1038
Copyright: © 2016 Takami Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Generally it is rare to select surgery for treating breast cancer liver metastases (BCLM), but we have carried out aggressively surgical treatment to BCLM. Especially as a part of treatment strategies, we have used intraoperative loco-regional microwave ablation, named microwave coagulo-necrotic therapy (MCN). In this study, we investigate whether or not surgical treatment, combining hepatic resection and/or MCN, is useful for treating BCLM.
Methods: Between 1994 and 2014, 33 patients with BCLM were surgically treated at National Hospital Organization Kyushu Medical Center by means of resection and/or MCN, followed by chemotherapy and/or endocrine therapy. The overall survival (OS) and disease-free survival (DFS) rates were determined.
Results: The mean number of hepatic metastases was 6.4, and the mean metastasis tumor diameter was 38.9 mm. The 1-, 3-, and 5-year survival rates of all patients after the first hepatic surgical treatment were 90.8%, 60.9%, and 53.8%, respectively. The mean survival time was 69.0 months.
In a univariate analysis of factors that may affect prognosis, the presence/absence of extrahepatic metastases was found to be the sole factor resulting in a significant difference. The number and sizes of hepatic metastases, and hormonal factors, were not found to be significant prognostic factors. Of 33 patients including patients, 7 survived with no recurrence of hepatic metastases for at least 3 years after hepatic metastatic surgery.
Conclusions: Our experience indicates that, surgery is an appropriate option for treating BCLM, and MCN in particular is a useful treatment for multiple liver metastases.
Keywords
Breast cancer liver metastases (BCLM), Microwave ablation, Microwave coagulo-necrotic therapy (MCN), Hepatic resection, Reduction surgery
Introduction
It is only in approximately 5% of breast cancer cases that hepatic metastases are restricted to the liver, and breast cancer liver metastases (BCLM) are therefore considered to constitute a systemic disease. In addition, numerous excellent chemotherapeutic agents are available. For these and other reasons, it is very rare for breast surgeons to select hepatic resection as a treatment method, despite resection of hepatic metastases being anatomically feasible [1-3].
Liver surgeons, on the other hand, are used to numerous hepatic metastases of other cancers, such as colorectal cancer. In addition, hepatic metastases, together with cerebral metastases of breast cancer, are considered to be life-threatening metastases and life-limiting factors, and they thus constitute a highly important factor with respect to prognosis. Therefore, liver surgeons tend to consider that hepatic resection contributes to improved prognosis when anatomically feasible [4]. There is thus a major gap between breast and liver surgeons' positions with respect to BCLM.
An additional point is that, at our center, favorable therapeutic results have been achieved with multiple as well as single hepatocellular carcinoma (HCC), albeit with only a small number of patients, by means of microwave coagulo-necrotic therapy (MCN) as well as resection [5]. Therefore, surgical treatment has been carried out at our center, because of the probability that surgical treatment combining hepatic resection and/or MCN is useful for treating multiple BCLM.
Surgery has been selected at our center for 33 patients with BCLM, the therapeutic results were evaluated, and the usefulness is reported here.
Patients and Methods
The number of patients treated for BCLM at Kyushu Medical Center, Deptartment of Hepatobiliary Pancreatic Surgery between the opening of the center, on July 1, 1994, and the end of 2014 was 36. The number of patients included in the present survey was 33, consisting of the above 36, except for 2 patients with whom surgery was judged to be feasible on the basis of preoperative diagnostic imaging, but multiple, intrahepatic micro-lymph-vessel metastases were found when laparotomy was carried out; and one patient with whom multiple hepatic micro metastases, which had not been shown by diagnostic imaging immediately beforehand at a different hospital, were found.
The surgical treatments used for BCLMare hepatic resection and MCN. Of these therapies, MCN is a loco-regional treatment method that has been used for HCC since the center was opened, involving the use of microwaves under laparoscopy, with laparotomy or small thoracotomy. The surgical procedure involves making multiple punctures with microwave antennas, guided by ultrasound images, from the vicinity of the HCC and toward the center, followed by microwave oscillation, and the details have been reported previously [5].
The criteria for selecting hepatic resection and/or MCN as the surgical treatment for BCLM are as follows:
(i) If the metastasis is superficial, and no more than 3 cm in diameter, or deep in the liver and no more than 2 cm in diameter, MCN is the first choice, whereas if it is in the hepatic margin, etc., resection is selected.
(ii) If small, multiple metastases are dispersed in both lobes of the liver, MCN is selected.
(iii) If there is a single metastasis 5 cm or more in diameter, or there are multiple, unevenly distributed metastases, resection is selected.
(iv) If at least one metastasis is 5 cm or more in diameter, and there are multiple metastases in both lobes of the liver, resection is carried out with the principal cancer nodules, and MCN is used for the remaining metastatic foci.
Even if extrahepatic metastases are also present, the resection/MCN exclusion criteria are not met if favorable local control of the extrahepatic metastases is achieved by means of chemotherapy and/or radiotherapy at the time of surgical treatment of the hepatic metastases.
The protocol for clinical evaluation of the usefulness of surgical treatment for BCLM was validated and approved by our center's Institutional Review Board. The surgery was carried out after explaining to the subjects that surgery is not the standard treatment for BCLM, and that a combination of systemic chemotherapy and multimodal therapy, etc., is required, and then obtaining their informed consent for participation. In addition, after treating the hepatic metastases, treatment was continued with supplementary chemotherapy, endocrine treatment, etc., primarily by the specialist physicians who introduced the patients. During the observation period at the center, the patients visited the center once every 3 months, and in the event of solely intrahepatic recurrence, options such as repeated surgery, and hepatic arterial infusion were looked into.
The Kaplan-Meier method was used for calculating the survival rate, and the log-rank test was used to compare survival rates. For all statistical values, the significant difference was taken to be P < 0.05.
Results
The ages of the 33 subjects at onset of breast cancer ranged from 27 to 70 years (mean, 46.2 years). The disease stages were 0, I, II, III, IV, and unknown, in 2, 6, 8, 6, 9, and 2subjects, respectively.
The hepatic metastases were metachronous in 27 subjects, and synchronous in 6. Excluding the synchronous cases, the period from breast cancer surgery to onset of hepatic metastases was 9 to 227 months (mean, 45.7 months). When the one patient with whom this period was 227 months (mean, 38.8 months), an unusually long time, was excluded.
After breast cancer surgery, adjuvant chemotherapy was carried out with 23 patients, and, among the 27 patients with metachronoushepatic metastases, with 11 patients neo-adjuvant therapy was carried out between discovery of the hepatic metastases and surgical treatment at the center. In addition, with 30 patients supplementary adjuvant therapy was carried out after surgical treatment of the hepatic metastases. There were only 10 patients with whom only intrahepatic metastases were found during the treatment period, and with the other 23 patients one type or another of extra hepatic metastases was also found. These extrahepatic metastases were treated with supplementary systemic adjuvant therapy, with radiotherapy being used for bone metastases, and resection and other treatments being used for ovarian metastases (Table 1).
![]()
Table 1: Characteristics of 33 patients with BCLM
View Table 1
The mean number of hepatic metastases was 6.4 (range, 1-31), and the mean tumor diameter was 38.9 mm (range, 12.3-120.0 mm).
The types of surgery selected were hepatic resection monotherapy, with 11 patients; MCN monotherapy, with 14 patients; and hepatic resection and MCN, with 8 patients. Of the 22 patients treated with either MCN monotherapy or resection and MCN, 16 had multiple (i.e. 2 or more) hepatic metastases and the mean number of metastases was 7.9. In addition, with 7 patients who showed recurrence of hepatic metastases, supplementary hepatic resection or MCN was carried out once or twice, and with 3such patients, anti-cancer agents were administered by hepatic arterial infusion.
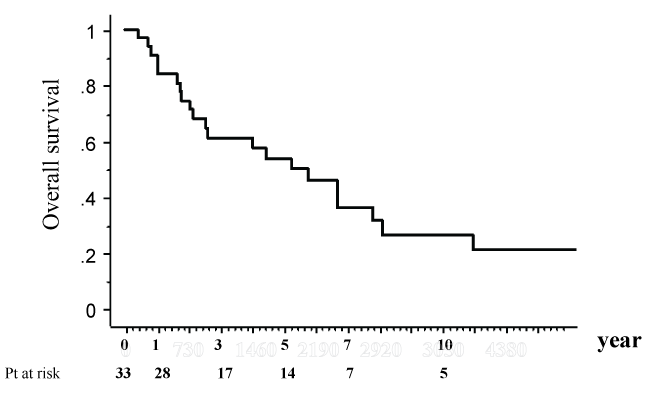
Of the 33 patients with BCLM, the cumulative survival rates after the initial surgery for the hepatic metastases were 90.8%, 60.9%, and 53.8%, respectively, after 1, 3, and 5 years (Figure 1). The mean survival time was 69.0 months.
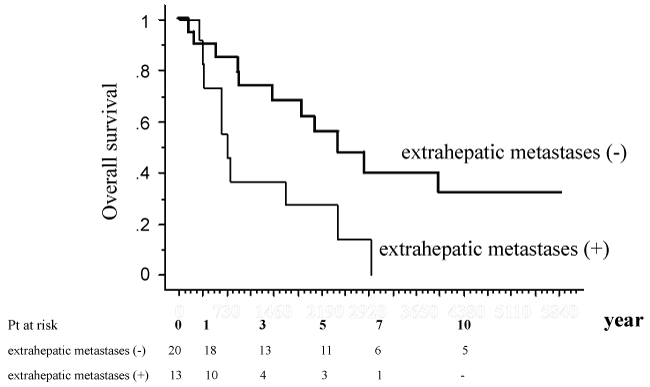
Univariate analysis was carried out to evaluate the factors contributing to cumulative survival rates after treatment of hepatic metastases, and various factors were found not to be significant, including age at breast cancer onset, age at hepatic metastasis onset, estrogen-receptor status, progesterone-receptor status, human epidermal growth factor receptor 2 (HER2) status, timing of hepatic metastasis, number of hepatic metastases, and sizes of hepatic metastases. The only parameter shown to be a significant prognostic factor was presence or absence of extra hepatic metastases at the time of onset of hepatic metastases (P = 0.0153; Table 2) (Figure 2).
.
Figure 2: Overall survival after initial treatment for the liver metastases based on status of the presence/absence of extra hepatic metastases at or before surgical treatment of hepatic metastases.
View Figure 2
![]()
Table 2: Univariate analysis of patient's characteristics for overall survival
View Table 2
With 7 of the 33 subjects, no recurrence of intrahepatic metastases was found for at least 3 years after surgical treatment of the hepatic metastases. Four of these subjects had single hepatic metastases, and these were treated by hepatic resection. The other 3subjects had multiple metastases (range, 5-17), and with two of these the treatment was MCN monotherapy, whereas with the other subject it was hepatic resection and MCN (Table 3).
![]()
Table 3: Cases with no recurrence in the liver for more than 3 years after initial treatment for liver metastases
View Table 3
Discussion
In comparison with breast cancer metastases in bone, lungs, etc., the survival time with hepatic metastases is very short, at 13 to 14.5 months, and, in common with cerebral metastases, hepatic metastases are therefore considered to be life threatening metastases, and to constitute a life limiting factor. They are thought to constitute an important factor affecting prognosis [1-3].
Breast cancer treatment methods have been hormone therapy, anti-HER2therapy, and chemotherapy, and marked improvements in response rate have been achieved using multi-drug chemotherapy. However, as the mean survival time is still only 25 to 29 months, and the 5-year survival rate is only 12.1%, the improvements in response rate cannot be said to be clearly reflected in the survival time [6,7].
In this context, we have come to consider whether it would be possible, with BCLM also, to improve treatment outcomes by surgical intervention, similar to that used with colorectal cancer hepatic metastases. At the same time, we are quite often asked by physicians whether MCN could not be used among patients suffering recurrence after breast cancer surgery, chemotherapy shows some but limited efficacy against bone and lung metastases, but no efficacy against hepatic metastases. Although it is of course recognized that BCLM is a systemic disease, considerable thought was given to treating hepatic metastases with drug therapy after the maximum possible volume reduction with appropriate use of MCN, a surgical technique of low invasiveness, and/or hepatic resection. And then the decision was made to carry out surgery, based primarily on MCN, for BCLM. However, possible difficulties with this treatment of BCLM that have been pointed out are that many breast cancer specialists will undoubtedly not accept this treatment, and its use is also likely to be queried at conferences, etc.
Nevertheless, the results of treatment of BCLMin the present survey show that prognoses were improved for numerous patients by means of surgical intervention. Similar findings in research at other clinical institutions have also previously been presented. For example, Pocard et al. carried out hepatic resection with 65 patients with BCLM, and found that the 3- and 4-year survival rates were 71 and 46% [8]. A second example is the study by Elias et al., who reported the 5-year survival and 5-year non-recurrence rates, with 44 BCLM patients, to be 34 and 22%, respectively [9]. A third example is the study by Adam et al., who found hepatic resection to be feasible with 85 of 108 (75%) BCLM patients, and reported the 5-year survival rate to be 37% [10]. Finally, Adam et al. also analyzed 454 BCLM patients, at 41 French clinical institutions, and showed the 5-year survival rate to be 41%, with BCLM patients being included in a favorable-prognosis group, together with kidney and ovary cancer hepatic metastasis patients, and in contrast with stomach cancer hepatic metastasis patients [4].
The favorable treatment outcome that we showed, a 5-year survival rate of 53.0%, was not solely due to hepatic resection, but also due to expansion of the indication of surgical treatment of multiple hepatic metastasis patients, together with energetic, concomitant application of MCN. We has previously reported that MCN has the capacity for local control of HCC, being at least the equal of hepatic resection in this respect [1], and it is considered that, for hepatic metastases also, ablation of individual metastases using microwaves offers the potential for local control of even multiple metastases to a degree at least equal to hepatic resection.
However, it is recognized that surgical treatment is volume reduction surgery, and an approach involving supplementary use of postoperative adjuvant chemotherapy is therefore preferable. Of the 33 subjects in the present research, 30 were supplementary treated with systemic chemotherapy, endocrine therapy, and/or molecular-targeted agents. Pocard et al. have proposed that, as surgical techniques, adjuvant surgery and surgical mass reduction should be used as aspects of multimodal treatment of breast cancer. In addition, Bathe et al. have presented an approach termed "cytoreductive strategy" [11]. Finally, Caralt et al. have presented an approach with a similar basis, but involving surgical intervention, termed" oncosurgical treatment" [12].
Once surgical treatment of BCLM has been judged to be feasible, this intervention should be carried out as soon as possible. We reported here the case of the 2 patients mentioned above with whom experimental laparotomy was carried out. Only 2 or 3 hepatic metastases were found on diagnostic images at that time. Although these patients had previously been administered chemotherapy and shown complete response, the patients experienced recurrence (repeated twice or 3 times), and it was therefore judged that control could not be achieved with chemotherapy, so the patients were referred to our department. When laparotomy was carried out with these patients, numerous sites of lymphatic infiltration were found in the micro-lymph-vessels of the liver surface. The suggested reason for this is that there is a fine lymph vessel network within the liver, and, even if BCLMare formed by transfer in the blood, the metastatic pattern of the original breast cancer, that is, the metastatic pattern mediated by the lymphatic system, is formed gradually during repetition of chemotherapy. On the basis of experience with these 2 patients, it is considered that surgical intervention for BCLM should probably be carried out before chemotherapy, if surgery is judged to be feasible. Similarly, Abbott et al. emphasized that it is important for chemotherapy to be effective before hepatic metastasis, and that the timing of chemotherapy before disease progression is thus important [13].
Among various factors, those that contribute to prognosis were evaluated. It has previously been reported that significant prognostic factors include age, hormone-receptor status, number of metastases, presence/absence of metachronoushepatic metastases, and R0 resection [14-16]. However, in the present research the only significant prognostic factor found was presence/absence of extra hepatic metastases before surgical treatment of hepatic metastases. The number of hepatic metastases, and the diameters of metastatic tumors were not found to affect survival, and it is therefore considered that, even in the case of patients with multiple hepatic metastases, more serious attention should be given to the option of surgical treatment, especially in the case of patients showing no extra hepatic metastases.
In summary, it is considered that the policy relating to BCLM should involve evaluating the efficacy of surgery, rather than sticking rigidly to systemic treatment.
Conclusions
In treating BCLM, favorable results were achieved with multimodal treatment, involving maximal volume reduction surgery by means of hepatic resection and MCN, and also postoperative adjuvant chemotherapy. MCN was particularly useful for treating multiple hepatic metastases.
Compliance with Ethical Standards
This study was not funded by any investor.
Conflict of Interest
Yuko Takami declare that I have no conflict of interest. Susumu Eguchi declares that he has no conflict of interest. Masaki Tateishi declares that he has no conflict of interest. Tomoki Ryu declares that he has no conflict of interest. Kazuhiro Mikagi declares that he has no conflict of interest. Yoshiyuki Wada declares that he has no conflict of interest. Hideki Saitsu declares that he has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
-
Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, et al. (2003) Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer 89: 284-290.
-
Pentheroudakis G, Fountzilas G, Bafaloukos D, Koutsoukou V, Pectasides D, (2006) et al. Metastatic breast cancer with hepatic metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500. Breast Cancer Res Treat 97: 237-244.
-
Eichbaum MH, Kaltwasser M, Bruckner T, de Rossi TM, Schneeweiss A, et al. (2006) Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat 96: 53-62.
-
Adam R, Chiche L, Aloia T, Elias D, Salmon R, et al. (2006) Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 244: 524-535.
-
Takami Y, Ryu T, Wada Y, Saitsu H (2013) Evaluation of intraoperative microwave coagulonecrotic therapy (MCN) for hepatocellular carcinoma: a single center experience of 719 consecutive cases. J Hepatobiliary Pancreat 20: 332-341.
-
Er O, Frye DK, Kau SW, Broglio K, Valero V, et al. (2008) Clinical course of breast cancer patients with metastases limited to the liver treated with chemotherapy. Cancer J 14: 62-68.
-
Duan XF, Dong NN, Zhang T, Li Q (2013) The prognostic analysis of clinical breast cancer subtypes among patients with liver metastases from breast cancer. Int J Clin Oncol 18: 26-32.
-
Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ (2001) [Hepatic resection for breast cancer metastases: results and prognosis (65 cases)]. Ann Chir 126: 413-420.
-
Elias D, Maisonnette F, Druet-Cabanac M, Ouellet JF, Guinebretiere JM, et al. (2003) An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg 185: 158-164.
-
Adam R, Aloia T, Krissat J, Bralet MP, Paule B, et al. (2006) Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 244: 897-907.
-
Bathe OF, Kaklamanos IG, Moffat FL, Boggs J, Franceschi D, et al. (1999) Metastasectomy as a cytoreductive strategy for treatment of isolated pulmonary and hepatic metastases from breast cancer. Surg Oncol 8: 35-42.
-
Caralt M, Bilbao I, Cortés J, Escartín A, Lázaro JL, et al. (2008) Hepatic resection for liver metastases as part of the "oncosurgical" treatment of metastatic breast cancer. Ann Surg Oncol 15: 2804-2810.
-
Abbott DE, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, et al. (2012) Resection of hepatic metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery 151: 710-716.
-
Dittmar Y, Altendorf-Hofmann A, Schüle S, Ardelt M, Dirsch O, et al. (2013) Liver resection in selected patients with metastatic breast cancer: a single-centre analysis and review of literature. J Cancer Res Clin Oncol 139: 1317-1325.
-
Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, et al. (2008) Liver resection for breast cancer metastasis: does it improve survival? Surg Today 38: 293-299.
-
Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, et al. (2010) Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol 17: 1546-1554.