International Journal of Cancer and Clinical Research
C4.4A and Aldehyde Dehydrogenase 1 (ALDH1) May Contribute to breast Cancer not Mediated Through epithelial-Mesenchymal Transition
Natsuko Inoue1, Takahiro Watanabe2, Seiichi Hirota2, Masahisa Ohtsuka3, Hirofumi Yamamoto3, Koji Morimoto4, Toyomasa Katagiri5 and Yasuo Miyoshi1*
1Department of Surgery, Division of Breast and Endocrine Surgery, Hyogo College of Medicine, Hyogo, Japan
2Department of Surgical Pathology, Hyogo College of Medicine, Hyogo, Japan
3Department of Gastroenterological Surgery, Graduate School of Medicine, Osaka University, Osaka, Japan
4Department of Human Life and Science, Osaka Women's Junior College, Osaka, Japan
5Division of Genome Medicine, Institute for Genome Research, University of Tokushima, Tokushima, Tokushima, Japan
*Corresponding author:
Yasuo Miyoshi, Department of Surgery, Division of Breast and Endocrine Surgery, Hyogo College of Medicine, Mukogawa 1-1, Nishinomiya City, Hyogo 663-8501, Japan, Tel: +81-798-45-6374, Fax: +81-798-45-6373, E-mail: ymiyoshi@hyo-med.ac.jp
Int J Cancer Clin Res, IJCCR-2-033, (Volume 2, Issue 5), Short Communication; ISSN: 2378-3419
Received: September 24, 2015 | Accepted: November 09, 2015 | Published: November 11, 2015
Citation: Inoue N, Watanabe T, Hirota S, Ohtsuka M, Yamamoto H, et al. (2015) C4.4A and Aldehyde Dehydrogenase 1 (ALDH1) May Contribute to breast Cancer not Mediated Through epithelial-Mesenchymal Transition. Int J Cancer Clin Res 2:033. 10.23937/2378-3419/2/5/1033
Copyright: © 2015 Inoue N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
C4.4A, a glycosylphosphatidyl-inositol-anchored membrane protein, plays an important role in epithelial-mesenchymal transition (EMT) affecting progression of colorectal cancer, while aldehyde dehydrogenase 1 (ALDH1) has been identified as a stem cell marker for breast cancer. Since cancer stem cells link to EMT phenotype, both molecules seem to be important for breast cancer progression mediated via EMT. In this study, associations between expressions of C4.4A or ALDH1 and vimentin, a mesenchymal marker, were immunohistochemically analyzed in 91breast cancer patients. Findings showed that 49 cancers (53.8%) were positive for C4.4A, 14 (15.4%) for ALDH1 and 12 (13.2%) for vimentin. C4.4A and ALDH1 positivity was significantly higher in the human epidermal growth factor receptor 2 (HER2)-positive (p = 0.014) and estrogen receptor (ER)-negative (p = 0.046) subsets, respectively. There was no significant association between C4.4A and ALDH1 expressions, in addition, C4.4A or ALDH1 expression show a no significant association with vimentin expression. These results suggest that C4.4A and ALDH1 make a subset-specific contribution to breast cancers not generated via EMT.
Keywords
Breast cancer, C4.4A, ALDH1, Epithelial-mesenchymal transition
Introduction
C4.4A, a glycosylphosphatidyl-inositol (GPI)-anchored membrane protein, has been isolated in a highly metastasizing tumor cell line [1]. In addition, C4.4A was found to play an important role in epithelial-mesenchymal transition (EMT) in colorectal cancers [2]. Since EMT links with metastatic processes, cancers with a high frequency of EMT seem to be likely to have an unfavorable outcome. In support of this speculation, it has been demonstrated that C4.4A correlates with poor prognosis for patients with colorectal, lung, and gastric cancers [3-5]. In contrast to these findings, however, we previously observed a favorable rather than an unfavorable disease-free survival rate in a subset of human epidermal growth factor receptor 2(HER2)-positive patients with C4.4A-positive breast cancers [6].
EMT has been characterized as expressing mesenchymal markers such as vimentin rather than epithelial markers such as E-cadherin and leading to an aggressive phenotype of breast cancer [7]. In addition, it has been demonstrated in a study of 126 breast cancer patients that Oct-4 and Nanog, which directly regulate the EMT and metastasis of breast cancer, are significantly associated with poor prognosis [8]. These findings seem to indicate that, unlike for other cancers, the involvement of C4.4A in breast cancer is not mediated through EMT.
In order to validate this notion, we analyzed associations between expressions of C4.4A and vimentin, a mesenchymal marker in breast cancers, since vimentin has been demonstrated to contribute to EMT through cytoskeletal organization and focal adhesion maturation [9]. Furthermore, aldehyde dehydrogenase 1 (ALDH1), identified as a cancer stem cell (CSC) marker and associated with poor prognosis of breast cancers [10], was also examined in order to clarify any links with C4.4A expressions.
Materials and methods
Tumor tissues and subtype classification of breast cancers
A total of 91 patients with invasive breast cancers operated at Hyogo College of Medicine between February 2005 and June 2011 were recruited for this study. Of the 385 breast cancer patients that were operated on during this period, those with non-invasive carcinomas (n = 68), those who had received chemotherapy (n = 47) or hormonal therapy (n = 18) before surgery, and those with synchronous bilateral breast cancers (n = 20) were excluded from this study. Of the remaining 232 cases, 91 whose samples included formalin-fixed and paraffin-embedded breast cancer tissues and included sufficient cancer cells were consecutively recruited. This was an immunohistochemical study using retrospectively collected storage samples, were stained for immunohistochemistry once after collection for ALDH1 and vimentin, but separately for C4.4A.
The Japanese Breast Cancer Society classification was used to determine the histologic classification and nuclear grade of the breast cancers [11]. Expressions of estrogen receptor (ER) and HER2 were determined by immunohistochemical staining and rated as positive if nuclear staining for ER was ≥ 1% or membrane staining for HER2 produced a score of 3 or 2 with a positive result for fluorescence in situ hybridization (FISH). Breast cancer subtypes were classified with a combination of ER and HER2 status.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded breast cancer tissues obtained from the enrolled patients were examined for C4.4A, ALDH1, and vimentin by means of immunohistochemical examination. Positive expressions in membrane and cytoplasm for C4.4A, and in cytoplasm for ALDH1 and vimentin were counted and recorded. Cut-off values were set at 10% for C4.4A [6], 5% for ALDH1 [12], and 1% for vimentin [13], on the basis of previously published findings. Moderate to intense staining in cancer cells was rated as positive for the 500 cancer cells in the stained lesions that were counted. The slides were examined by two investigators (N.I., T.W.) who were unaware of the clinicopathologic features of the patients.
The antibodies used in this study, C4.4A [6], ALDH1 [14] and vimentin [15], have been described elsewhere. Briefly, antigen was retrieved by heating at 98 °C for 20 min in 10 mM citrate buffer (pH = 6.0) for C4.4A and ALDH1 or heating at 98 °C for 10 min in Tris/EDTA buffer (pH = 9.0) for vimentin. Next, the sections were treated with REAL peroxidase-blocking solution (DAKO, Glostrup, Denmark) at room temperature for 20 min to inhibit endogenous peroxidase activity, followed by treatment with the primary antibodies incubated at 4 °C overnight. Finally, the sections were incubated with HRP polymer (Envision Plus Kit; DAKO) at room temperature for 30 min and the peroxidase reaction was visualized with a 3, 3-diaminobenzidine-tetrahydrochloride solution (DAKO) according to the manufacturer's protocol, followed by counterstaining with hematoxylin.
Statistical Analysis
Differences in clinicopathological characteristics between tissues rated positive and negative for C4.4A, ALDH1 or vimentin were calculated with the chi square or Fisher's exact test as appropriate. Correlations between C4.4A, ALDH1 or vimentin status were also calculated with the Fisher's exact test. Differences were considered statistically significant if p < 0.05. JMP Pro 10 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Correlations of C4.4, ALDH1 or vimentin expression levels with clinicopathological characteristics
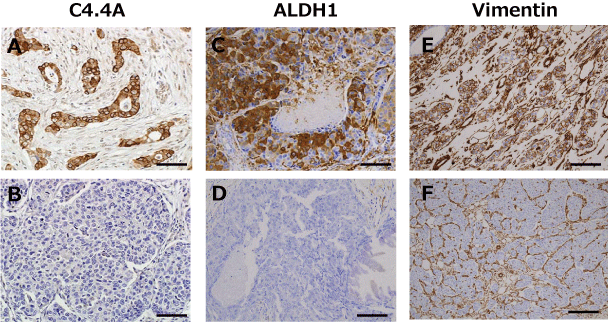
Representative results of C4.4A, ALDH1, and vimentin immunostaining are displayed in figure 1. Positive expressions in membrane and cytoplasm for C4.4A (Figure 1A), in cytoplasm for ALDH1 (Figure 1C) and vimentin (Figure 1E). Positivity was determined based on the percentage of positive cancer cells. As shown in table 1, the number of C4.4A-positive cancers in the HER2-positive subset was significantly higher as compared with those in the HER2-negative subset (78.9% vs 47.2%, p = 0.014). ALDH1 positivity was significantly higher (p = 0.041) for tumors measuring > 2 cm (29.6%) than for those ≤ 2 cm in size (9.5%). Furthermore, the number of ALDH1-positive cancers was significantly higher in the ER-negative than in the ER-positive subset (29.2% vs 10.4%, p = 0.046). Vimentin expression showed significant associations with higher nuclear grade (p = 0.0016), ER-negativity (p = 0.0002) and progesterone receptor negativity (p = 0.0025).
.
Figure 1: Representative immunestaining for C4.4A (A, B), aldehyde dehydrogenase 1 (ALDH1) (C, D), and vimentin (E, F). Positive (A, C, E) and negative (B, D, F) staining is shown. Positivity was determined according to the percentage of positive cancer cells in membrane and cytoplasm for C4.4A, and in cytoplasm for ALDH1 and vimentin as described in the Materials and Methods section. Scale bars represent 100 μm.
View Figure 1
![]()
Table 1: Correlations of C4.4A, ALDH1*1 and vimentin expression levels with clinicopathological characteristics.
View Table 1
Correlation between C4.4A and ALDH1 or vimentin expression
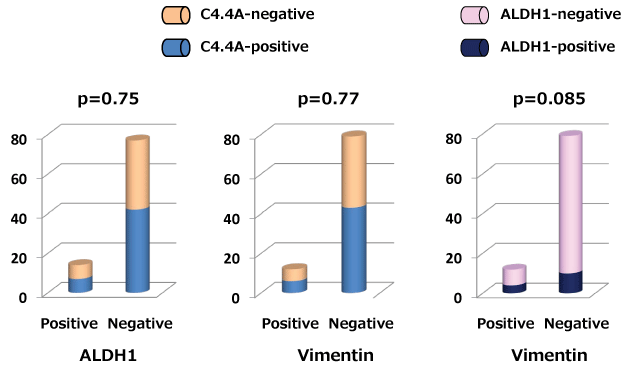
ALDH1 expression was marginally but not significantly associated with vimentin expression (p = 0.085), and there was no significant correlation between C4.4A and vimentin expressions (p = 0.77) as shown in figure 2. In addition, C4.4A and ALDH1 expression showed no significant association (p = 0.75).
.
Figure 2: Correlation between C4.4A and aldehyde dehydrogenase 1 (ALDH1) or vimentin expressions is shown as a column bar graph. Correlation between ALDH1 and vimentin is also represented as a column bar graph.
View Figure 2
C4.4A, ALDH1 and vimentin expression levels in breast cancer subtypes
We were able to establish that expression of C4.4A was significantly higher in the HER2-positive and of ALDH1 in the ER-negative subset (Table 1). Next, correlations of C4.4A, ALDH1 and vimentin expression levels with breast cancer subtype were further analyzed with the results shown in table 2. Expression of all three markers showed statistically significant associations with breast cancer subtypes (p < 0.0001 for C4.4A, p = 0.034 for ALDH1, p = 0.001 for vimentin). Only one (6.7%) out of 15 ER-negative/HER2-negative breast cancers was positive for C4.4A and ALDH1 positivity was rarely detected in the ER-positive/HER2-negative subset (8.8%). On the other hand, vimentin expression was higher for the ER-negative subset (ER-negative/HER2-positive: 33.3%, ER-negative/HER2-negative: 40%) and lower for the ER-positive subset (ER-positive/HER2-negative: 5.3%, ER-positive/HER2-positive: 0%), irrespective of HER2 status.
![]()
Table 2: C4.4A, ALDH1*1 and vimentin expression levels in subtypes.
View Table 2
Discussion
According to our findings presented here, the fact that there was no significant association between C4.4A and vimentin expressions suggests that C4.4A performs amediating function in breast cancers not through EMT, but through other mechanisms. This mechanism may explain, at least partly, the reason why our previous study found that prognosis was not poorer for the C4.4A-positive subset [6]. Initially, it was found that C4.4A promoted EMT at the invasive front of colorectal cancer [2], but another function of C4.4A was recently detected, namely, that C4.4A recruits and cooperates with alpha6beta4, and via this protein with matrix metalloproteinase (MMP) 14, to trigger matrix degradation and PI3K/Akt activation [16]. Thus, C4.4A is not necessarily involved in invasion and metastasis, but it may be conceivable that other mechanisms like those described above contribute to the progression of breast cancers.
ALDH1 is a well-established marker for cancer stem cells in the breast. Although cancer stem cells have the potential to play a role in EMT, one issue concerning the relationship between ALDH1-positive cells and the EMT phenotype has yet to be fully clarified. According to Liu et al. study of primary breast cancer cells, EMT-associated genes including vimentin were significantly enhanced in populations positive for CD24- CD44+, another cancer stem cell marker, but reduced in an ALDH1-positive population [17]. The results obtained in our study, combined with those reported by Liu et al., seem to suggest that ALDH1 action in breast cancer progression may also, at least partly, be mediated not through EMT but through another mechanism. An interesting finding of our study was that C4.4A was rarely expressed in the ER-negative/HER2-negative subset, while little positivity for ALD H1 was detected in the ER-positive/HER2-negative subset. These findings point to the possibility that these molecules may make a subtype-specific contribution to the development of breast cancer, although the details of such a mechanism remain unknown. Our study therefore needs to be validated in a future study including a larger number of samples as well as other factors involved in EMT or cancer stem cells.
In conclusion, we were able to demonstrate that neither C4.4A nor ALDH1 is associated with vimentin expression. Furthermore, expressions of C4.4A and ALDH1 were found to be enhanced in HER2-positive and ER-negative subsets, respectively. These results seem to indicate a potential subset-specific role of these molecules in breast cancers not mediated via EMT. Since biology of breast cancers differs depending on the subtype, analyses of subtype-specific molecules associated with EMT as well as CSC may be important. Our data presented in this study, may therefore provide useful information for a better understanding of this issue.
Acknowledgements
This study was funded by the Grant of Hyogo College of Medicine.
Conflict of Interest
The all authors of this manuscript have no conflict of interest.
References
-
Rosel M, Claas C, Seiter S, Herlevsen M, Zoller M (1998) Cloning and functional characterization of a new phosphatidyl-inositol anchored molecule of a metastasizing rat pancreatic tumor. Oncogene 17: 1989-2002.
-
Oshiro R, Yamamoto H, Takahashi H, Ohtsuka M, Wu X, et al. (2012) C4.4A is associated with tumor budding and epithelial-mesenchymal transition of colorectal cancer. Cancer Sci 103: 1155-1164.
-
Konishi K, Yamamoto H, Mimori K, Takemasa I, Mizushima T, et al. (2010) Expression of C4.4A at the invasive front is a novel prognostic marker for disease recurrence of colorectal cancer. Cancer Sci 101: 2269-2277.
-
Hansen LV, Skov BG, Ploug M, Pappot H (2007) Tumour cell expression of C4.4A, a structural homologue of the urokinase receptor, correlates with poor prognosis in non-small cell lung cancer. Lung Cancer 58: 260-266.
-
Cheng DQ, Gu XD, Li ZY, Xiang JB, Chen ZY (2014) Expression of C4.4A is a potential independent prognostic factor for patients with gastric cancer. Asian Pac J Cancer Prev 15: 3895-3899.
-
Miyake T, Ito T, Yanai A, Inoue N, Miyagawa Y, et al. (2015) C4.4A highly expressed in HER2-positive human breast cancers may indicate a good prognosis. Breast Cancer 22: 366-373.
-
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, et al. (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68: 989-997.
-
Wang D, Lu P, Zhang H, Luo M, Zhang X, et al. (2014) Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget 5: 10803-10815.
-
Liu CY, Lin HH, Tang MJ, Wang YK (2015) Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 6: 15966-15983.
-
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, et al. (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1: 555-567.
-
Sakamoto G, Inaji H, Akiyama F, Haga S, Hiraoka M, et al. (2005) General rules for clinical and pathological recording of breast cancer 2005. Breast Cancer 12 Suppl: S1-27.
-
de Beca FF, Caetano P, Gerhard R, Alvarenga CA, Gomes M, et al. (2013) Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol 66: 187-191.
-
Liu T, Zhang X, Shang M, Zhang Y, Xia B, et al. (2013) Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. J Surg Oncol 107: 188-194.
-
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, et al. (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15: 4234-4241.
-
Gerhard R, Ricardo S, Albergaria A, Gomes M, Silva AR, et al. (2012) Immunohistochemical features of claudin-low intrinsic subtype in metaplastic breast carcinomas. Breast 21: 354-360.
-
Thuma F, Ngora H, Zoller M (2013) The metastasis-associated molecule C4.4A promotes tissue invasion and anchorage independence by associating with the alpha6beta4 integrin. Mol Oncol 7: 917-928.
-
Liu S, Cong Y, Wang D, Sun Y, Deng L, et al. (2013) Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2: 78-91.





