International Journal of Cancer and Clinical Research
Vaginal Malignant Peripheral Nerve Sheath Tumor (MPNST) With Unusual Liposarcomatous Differentiation - A Case Report
F Gougeon1*, J. Doyon2, P. Sauthier3 and K. Rahimi1
1Centre universitaire de l'universite de Montreal, departement de pathologie, Montreal, Canada
2Centre de sante Maisonneuve-Rosemont, departement de pathologie, Montreal, Canada
3Centre universitaire de l'universite de Montreal, departement de gyneco-oncologie, Canada
*Corresponding author:
Francois Gougeon, Centre universitaire de l'universite de Montreal, departement de pathologie, Montreal, Canada, Tel: 4388830570, E-mail: francoiswgougeon@gmail.com
Int J Cancer Clin Res, IJCCR-2-030, (Volume 2, Issue 4), Case Report; ISSN: 2378-3419
Received: September 25, 2015 | Accepted: October 26, 2015 | Published: October 28, 2015
Citation: Gougeon F, Doyon J, Sauthier P, Rahimi K (2015) Vaginal Malignant Peripheral Nerve Sheath Tumor (MPNST) With Unusual Liposarcomatous Differentiation - A Case Report. Int J Cancer Clin Res 2:030. 10.23937/2378-3419/2/4/1030
Copyright: © 2015 Gougeon F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are rare sarcomas usually arising in peripheral nerve bundles or from pre-existing neurofibromas. They have frequent divergent differentiation. Here we present a case of MPNST arising in the vagina of a 70 y.o women. Beside the unusual location, this tumor presented liposarcomatous differentiation, a finding which has only been reported three times in the past and never in a MPNST of the female genital tract.
Keywords
MPNST, Vagina, Sarcoma, Liposarcomatous
Introduction
Malignant peripheral nerve sheath tumors (MPNST) are neoplasms that arise from a peripheral nerve or, when situated in extra-neural soft tissue, that show nerve sheath differentiation. They account for about 5 - 10% of soft tissue sarcomas and are strongly associated with neurofibromatosis type I. Most of these tumors arise either from a pre-existent neurofibroma or de novo from nerve sheath. The most frequent locations are the large and medium nerves of the buttock and thighs or brachial plexus. A small minority arises from extra-neural soft tissue [1].
Approximately 15% of MPNSTs show divergent histological differentiation, either mesenchymal or epithelial. Divergent mesenchymal differentiation usually consists of cartilage, skeletal muscle or bone. We are aware of only three reported cases of MPNST which presented liposarcomatous differentiation [2-4].
Although MPNSTs have been reported in many locations unrelated to major nerve trunks, there is, to date, only two cases of vaginal MPNST reported in the literature [5,6].
Clinical
The patient is a 70 y.o. women of French-Canadian ancestry in good general health. There was no personal or family history of neurofibromatosis.
She was referred to the gynecological-oncology clinic for spotting which had started a year before. Two months prior to presentation, she noticed an enlarging vaginal mass, as well as urinary symptoms and loss of around 10 pounds.
A PET-SCAN showed two vaginal masses measuring 8.6 cm and 6.0 cm. One of them showed cavitation. Two lymph nodes adjacent to the left iliac vessels were suspicious for metastasis. There was no evidence of extra-nodal metastasic disease.
The patient underwent a surgical biopsy of one of the vaginal masses. The diagnosis of MPNST, epithelioid variant with liposarcomatous differentiation, grade 3/3 was made and confirmed by a pathologist specialized in soft-tissue tumors.
The patient was offered neo-adjuvant radio-chemotherapy and surgery but chose a palliative approach.
Pathology
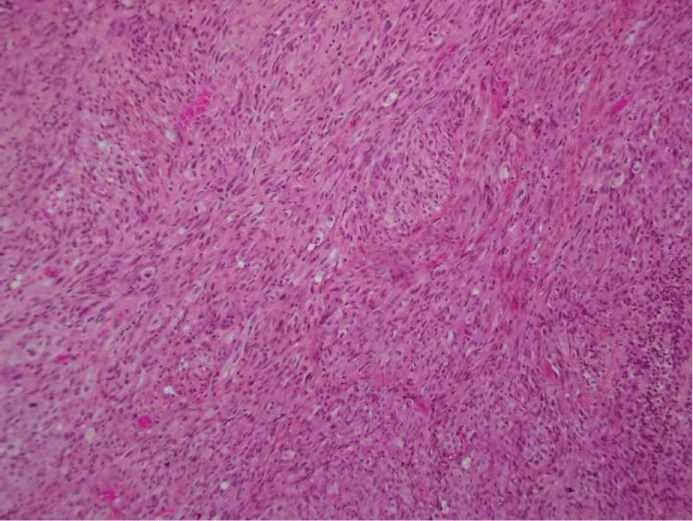
Microscopic evaluation revealed an ulcerated lesion consisting of spindled and epithelioid neoplastic cells with marked atypia, pleomorphism and high mitotic activity (20-25/10 hpf). Irregularly shaped vessels of various sizes were unevenly distributed throughout the lesion. The malignant cells showed hyperchromatic nuclei with one to three prominent eosinophilic nucleoli and vacuolated amphophilic to eosinophilic cytoplasm (Figure 1).
.
Figure 1: Tumor consisted of atypical epithelioid cells with abundant eosinophilic cytoplasm (HE, 100x)
View Figure 1
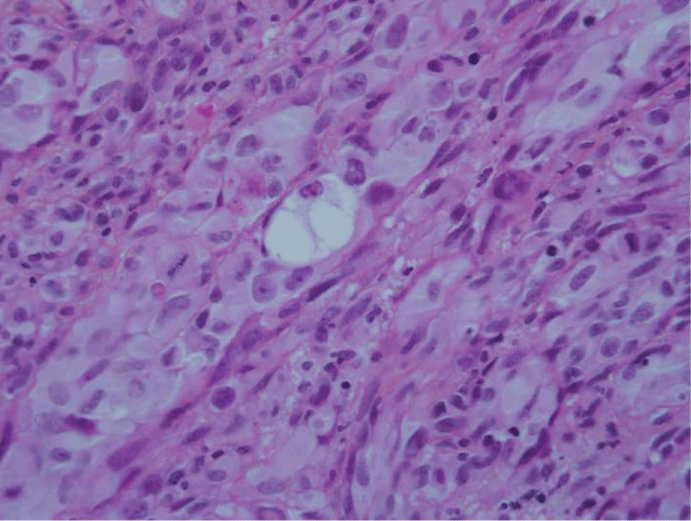
Some cells showed optically clear vacuoles that sometimes pushed the nuclei to the periphery. These vacuoles were not stained by Alcian blue or hyaluronidase. These cells appeared frankly neoplastic (Figure 2).
.
Figure 2: Some cells showed optically clear vacuoles which did not stain for alcian blue or hyaluronidase (HE, 200x)
View Figure 2
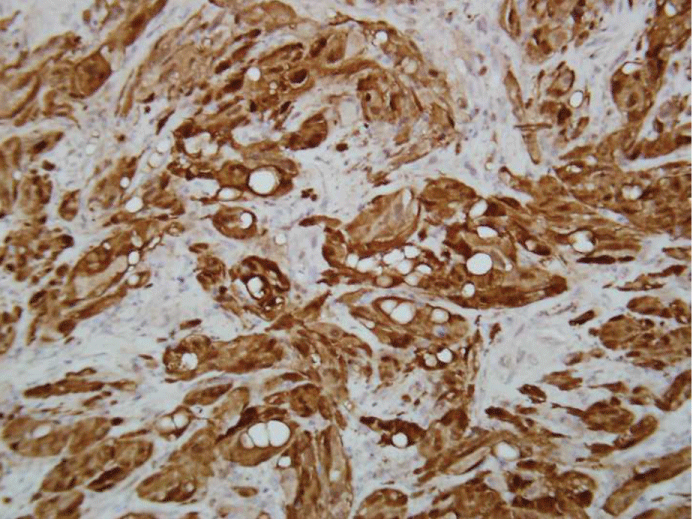
A strong nuclear and cytoplasmic reaction was obtained with Immunohistochemistry for S-100 protein (Figure 3). Focal staining was present with smooth muscle actin and calretinin. CD 99 was also diffusely positive. A strong and diffuse staining for vimentin was present. The neoplastic cells showed no staining for the following antibodies: caldesmon, desmin, myogenin, CD31, CD34, HMB45, MART-1, Melan-A, CD10, inhibin, WT1, p16, estrogen receptor, progesterone receptor, p63, EMA, cytokeratin cocktail AE1/AE3 and cytokeratin 7, cytokeratin 20, cytokeratin 34BE-12, cytokeratin 5/6, cytokeratin 8/18, DOG-1, MDM2 and CDK4 (Table 1). There was weak and focal staining for CD117. About 80% of neoplastic cells were positive for p53. Ki67 immunostaining showed a very high proliferation rate.
![]()
Table 1: Antibody list
View Table 1
Discussion
MPNSTs are defined as malignant neoplasms derived from peripheral nerves or showing nerve sheath differentiation (excluding tumors arising from the peripheral nerve vasculature or epineurieum) [7]. They represent about 5 - 10% of soft tissue sarcomas [1]. They are very aggressive tumors, frequently showing early haematogenous dissemination [8]. Most of these tumors are high grade and measure more than 5 cm at the time of diagnosis. They are associated with neurofibromatosis type 1 in about a third of patients. The 5-year survival rate for treated patients has historically been reported to be less than 50% but seems to have improved in recent years due to improved surgical techniques and liberal use of neo-adjuvant treatments [8,9].
Pathologically, although there is good agreement on diagnosis for MPNSTs arising from benign neural tumors or de-novo from nerve bundles, there is more discordance for tumors arising sporadically in other locations. The major histological criteria are: 1) The presence of dense and hypodense fascicles alternating in a marbled appearance 2) Asymmetrically tapered spindle cells with irregular buckled nuclei and 3) Schwann cell differentiation by immunohistochemistry or electron microscopy in a sarcomatous tumor. Other weaker criteria include nuclear palisading, whorled structures and hyperplastic vascular changes [10].
Our case is unusual because of its location and because of the liposarcomatous differentiation. Although a small number of cases have been reported in the uterine cervix and vulva, we are aware of only two reported cases of vaginal MPNST [5,6,11,12].
A recent retrospective series of 175 cases showed that the most frequent localizations of MPNSTs are the extremities (45%) followed by the trunk (34%) and head and neck region (19%) [7]. They usually arise from large or medium nerve trunks or pre-existing neurofibromas [13]. Unusual localizations of MPNSTs that have been reported include the kidney, the oral cavity, the eyelids and the heart [14-17].
As a whole, vaginal sarcomas represent about 3% of vaginal malignancies. Roughly two thirds of those (2/3) are leiomysarcomas. The differential diagnosis of spindle cell tumors of this region also includesstromal sarcomas and malignant mixed Mullerian tumors.Embryonal rhabdomyosarcomas occur mainly in children. Most of the patients with vaginal sarcomas present with vaginal discharge or palpable mass [18].
Divergent differentiation is a well- known phenomenon in MPNSTs. A review of the histological characteristics of 120 cases showed this occurred in about 16% of MPNSTs [8]. Rhabdomyoblastic, osteoblastic, chondroblastic and glandular differentiation have been described. Three cases of liposarcomatous differentiation have been reported [2-4]. A few cases of benign adipocyte differentiation have been reported in benign nerve sheath tumors [19]. It is important not to mistake benign adipocytes entrapped by the tumor asliposarcomatous differentiation. The cells showing liposarcomatous differentiation should have atypical pleomorphic nuclei, as opposed to those of entrapped adipocytes. In our case, the adipocytes showed frank atypia, and were thus considered malignant. This type of divergent differentiation can also raise the possibility of a de-differentiated liposarcoma, but the de-differentiated component of liposarcomas is usually high-grade "malignant fibrous histiocytoma-like", which was not the case here [2]. Furthermore, de-differentiated liposarcomas usually have positive staining for MDM-2 and CDK-4 [20].
The differential diagnostic in this case also included other types of sarcomas (leiomyosarcoma, fibrosarcoma) and spindle cell melanoma. Other sarcomas were ruled out mainly by immunochemistry. The lack of expression of myogenic markers (desmin, caldesmon) and cytokeratins argued against a diagnosis of leiomyosarcoma or synovial sarcoma, respectively. The histological appearance would also have been highly atypical for synovial sarcoma. De-differentiated liposarcoma was ruled-out on the basis of negative staining for MDM-2 and CDK-4. Malignant melanoma was ruled-out on the basis of negative melanocytic markers other than S-100 (HMB45, Melan-A, Mart-1).
Conclusion
In summary, we present here a case of vaginal MPNST, epithelioid type, with liposarcomatous differentiation. A review of the literature shows that these tumors are extremely rare in the female genital tract. It is a reminder that these tumors can arise at various locations beside major nerve trunks. Liposarcomatous differentiation has been described in very few cases, although MPNSTs are notorious for having divergent differentiation, usually rhabdomyosarcomatous or osteoblastic.
References
-
Danid N, Hiroko O, Otmar D (2007) WHO classification of tumors-pathology and genetics of tumors of the nervous system.
-
Tirabosco R, Galloway M, Bradford R, O'Donnell P, Flanagan AM (2010) Liposarcomatous differentiation in malignant peripheral nerve sheath tumor: a case report. Pathol Res Pract 206: 138-142.
-
Guo A, Liu A, Wei L, Song X (2012) Malignant peripheral nerve sheath tumors: differentiation patterns and immunohistochemical features - a mini-review and our new findings. J Cancer 3: 303-309.
-
Devadoss CW, Rau AR, Manjari S, Hasaf MK (2014) Paediatric malignant peripheral nerve sheath tumor with osteoid, rhabdomyosarcomatous, and liposarcomatous differentiation. J Postgrad Med 60: 348-349.
-
Mhaskar R, Mhaskar V, Pritilata R (2009) Malignant peripheral nerve sheath tumour of the vagina. J Obstet Gynaecol 29: 360.
-
Zhang J, Sun Y, Peng ZL (2009) Malignant peripheral nerve sheath tumor of the vagina. Saudi Med J 30: 705-707.
-
Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, et al. (2012) Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol 19: 878-885.
-
Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM (1986) Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57: 2006-2021.
-
Minovi A, Basten O, Hunter B, Draf W, Bockmuhl U (2007) Malignant peripheral nerve sheath tumors of the head and neck: management of 10 cases and literature review. Head Neck 29: 439-445.
-
Enzinger F, Weiss S (2008) Malignant tumors of the peripheral nerves. In: Soft Tissue Tumors. 3rd ed. St-Louis MI: Mosby.
-
Ozdal B, Oz M, Korkmaz E, Ataoglu O, Gungor T, et al. (2014) Malignant peripheral nerve sheath tumor of the vulva, an unusual differential diagnosis for vulvar mass. Int J Surg Case Rep 5: 793-795.
-
Panigrahi S, Mishra SS, Das S, Dhir MK (2013) Primary malignant peripheral nerve sheath tumor at unusual location. J Neurosci Rural Pract 4: S83-86.
-
Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM (2005) Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 65: 205-211.
-
Khandelwal A, Gupta A, Khandelwal K (2011) Malignant peripheral nerve sheath tumor of kidney. Iran J Kidney Dis 5: 373.
-
Ramalingam WV, Nair S, Mandal G (2012) Malignant peripheral nerve sheath tumor of the oral cavity. J Oral Maxillofac Surg 70: e581-585.
-
Cho WC, Jung SH, Lee SH, Bang JH, Kim J, et al. (2012) Malignant peripheral nerve sheath tumor arising from the left ventricle. J Card Surg 27: 567-570.
-
Lindsay RA, Gupta D, Keene CD, Bhrany AD, Chang SH (2015) Malignant Peripheral Nerve Sheath Tumor of the Lower Eyelid: Case Presentation and Literature Review. Ophthal Plast Reconstr Surg .
-
Magne N, Pacaut C, Auberdiac P, Jacquin JP, Chargari C, et al. (2011) Sarcoma of vulva, vagina and ovary. Best Pract Res Clin Obstet Gynaecol 25: 797-801.
-
Plaza JA, Wakely PE, Jr., Suster S (2006) Lipoblastic nerve sheath tumors: report of a distinctive variant of neural soft tissue neoplasm with adipocytic differentiation. Am J Surg Pathol 30: 337-344.
-
Coindre JM, Pedeutour F, Aurias A (2010) Well-differentiated and dedifferentiated liposarcomas. Virchows Arch 456: 167-179.