International Journal of Cancer and Clinical Research
Smac13-Tat Fusion Peptide Induces Cell Death and Sensitizes HeLa Cells to Chemotherapeutic Drugs
Yoshinori Mano, Toshihiko Torigoe*, Hiroko Asanuma, Yoshihiko Hirohashi and Noriyuki Sato
Department of Pathology, Sapporo Medical University School of Medicine, Japan
*Corresponding author:
Toshihiko Torigoe, Associate Professor, Department of Pathology, Sapporo Medical University School of Medicine, S-1, W-17, Chuo-ku, Sapporo 060-8556, Japan, Tel: 81-11-611-2111, Fax: 81-11-643-2310, E-mail: torigoe@sapmed.ac.jp
Int J Cancer Clin Res, IJCCR-2-010, (Volume 2, Issue 1), Original article; ISSN: 2378-3419
Received: January 09, 2015 | Accepted: January 16, 2015 | Published: January 19, 2015
Citation: Mano Y, Torigoe T, Asanuma H, Hirohashi Y, Sato N (2015) Smac13-Tat Fusion Peptide Induces Cell Death and Sensitizes HeLa Cells to Chemotherapeutic Drugs. Int J Cancer Clin Res 2:010. 10.23937/2378-3419/2/1/1010
Copyright: © 2015 Mano Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The inhibitor of apoptosis proteins (IAPs) are overexpressed in a variety of cancer cells and play an important role in the inhibition of caspases, thereby suppressing programed cell death and leading to chemoresistance of cancer cells. However, the anti-apoptotic function of IAPs could be suppressed by Smac, the second mitochondria-derived activator of caspase through its direct interaction with IAPs. Smac can interact with IAPs through the N-terminal Ala-Val-Pro-Ile tetrapeptide domain.
Methods: In the present study, the first 7 amino acid sequence (residues 54-60), Smac7, and the first 13 amino acid sequence (residues 54-66), Smac13, which are conserved among mammalian homologues were transduced into cells by bonding to the HIV-1 Tat-derived cell-penetrating peptide.
Results: The Smac-Tat peptides induced cell death and increased the sensitivity to chemotherapeutic agents in HeLa cells, whereas they did not affect cell survival in normal lymphocytes. The Smac7-Tat peptide could bind to Livin but not Survivin.
Conclusions: We demonstrate here that Smac13-Tat is more effective for chemosensitization than Smac7-Tat and that its use might serve as a potent molecular targeted therapy for chemoresistant cervical cancer.
Keywords
Smac, Survivin, Livin, Drug resistance, Cervical cancer
Abbreviations
IAP: Inhibitor of Apoptosis Protein; Smac: The Second Mitochondria-Derived Activator of Caspase; HIV: Human Immunodeficiency Virus; BIR: Baculovirus IAP Repeat; PBL: Peripheral Blood Lymphocyte; MTT: 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl tetrazolium bromide
Introduction
The inhibitor of apoptosis proteins (IAPs) are highly conserved anti-apoptotic proteins in various organisms ranging from yeast to mammals [1,2]. They have one or more structurally conserved domains, termed baculovirus IAP repeat (BIR) domains, which mediate the interaction with caspases. Thus far, eight human IAP members have been reported: NAIP, c-IAP1, c-IAP2, XIAP, ILP-2, Apollon, Survivin, and Livin [3]. Most of them also contain a RING finger domain that functions as E3 ubiquitin ligase [1,2] and promote proteasome-mediated degradation of caspases. Thus, they are endogenous signaling molecules that have important roles for the regulation of apoptotic pathways in normal cells. More importantly, XIAP, Survivin and Livinare are frequently over expressed in various tumor cells and cause resistance against chemotherapeutic agents and radiation [4-8]. Many reports have shown that their expression is correlated with tumor aggressiveness [9-12]. Though XIAP is expressed in normal cells and tissues, expression of Livin and Survivin is limited in most normal adult tissues [8,13]. These findings have led to great interest in Livin and Survivin as ideal therapeutic targets for cancer.
It has been reported that the expression and functions of IAP members are regulated by mitochondrial proteins such as Smac (the second mitochondria-derived activator of caspase) and HtrA2 [14]. Smac contains a mitochondria-targeting sequence at its N-terminus, which is cleaved after mitochondrial entry [15]. Upon apoptotic signals, it is released into the cytosol and binds to a BIR domain of IAP through the N-terminal Ala-Val-Pro-Ile (AVPI) tetrapeptide domain, thereby leading to inhibition of their interaction with caspases. In this context, Smac is a kind of endogenous IAP antagonist. Furthermore, it has been reported that a seven amino-acid N-terminal peptide of cleaved Smac (Smac7, AVPIAQK) is necessary and sufficient to bind to IAP [16]. Studies have shown that synthetic Smac7 peptide is transduced into cells by generating a fusion peptide through bonding of the C-terminus of the Smac7 peptide to the N-terminus of a cell-penetrating peptide such as the antennapedia peptide, polyarginine peptide or HIV-Tat peptide, and successfully induces apoptosis in tumor cells [17-20]. Since the 13 N-terminal amino acids of cleaved Smac are highly conserved among mammalian Smac proteins [21], it was speculated that the Smac13-Tat peptide might have higher capacity to induce cell death of cancer cells than Smac7-Tat.Therefore, in the present study, we analyzed the efficiency of the Smac13-Tat peptide for inducing cell death and sensitization to chemotherapeutic drugs. HeLa cells were examined because they express both Survivin and Livin and have resistance to drug-induced cell death. We examined the chemosensitivity to VP-16 and paclitaxel, which are known as a topoisomerase inhibitor and an antimicrotubule agent, respectively. Since mutation of the very first amino acid Ala of Smac7 to Met leads to loss of the interaction with IAPs [15,22,23], we examined Smac7M-Tat and Smac13M-Tat as control peptides. In addition, the target IAP molecule of Smac7-Tat was defined in transfected cells.
Materials and Methods
Cell culture
The experiments were conducted with the approval of the Sapporo Medical University Study Review Board. Human cervical cancer HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma- Aldrich, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 units/mL penicillin G, and 100 µg/mL streptomycin in a 5% CO2 incubator at 37°C. Human peripheral blood lymphocytes were collected from a healthy volunteer and cultured in RPMI1640 medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% FBS in a 5% CO2 incubator at 37ºC.Human embryonal kidney HEK293 cells and HEK293T cells were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in DMEM containing 10% FBS.
Synthetic peptides
The amino acid sequences of the synthetic peptides are listed in Table 1. Smac7 (AVPIAQK) and Smac13 (AVPIAQKSEPHSL) are 7 amino acidand 13 amino acid N-terminal sequences of the cleaved human Smac protein, respectively. Tat (YGRKKRRQRRR) is a cell-penetrating peptide derived from HIV-Tat protein [24-26]. All of these synthetic peptides were purchased from SigmaGenosys (Ishikari, Japan).Mean fluorescence intensity of cells with FITC-labeled peptide was analyzed using ImageJ software (Dr. Wayne Rasband, the Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA).
![]()
Table 1: Amino acid sequences of the peptides used in the study
View Table 1
Cell survival assay.
The cell survival rate was assessed using the MTT colorimetric assay. It is based on cleavage of the tetrazolium salt MTT to formazan by cellular mitochondrial dehydrogenases. With this assay, a decrease in the number of viable cells results in a decrease in the overall activity of the mitochondrial dehydrogenases in the sample. The decrease in the enzyme activity leads to a decrease in the formazan dye formed, which can be quantified by using a plate reader. Briefly, cells were suspended at a cell density of 2x104 cells in 100 µL culture medium per well on 96-well flat-bottom plates (Corning, NY, USA) and incubated with the peptides and/or chemotherapeutic reagents for the indicated time at 37ºC. Then, the cells were incubated with MTT reagent (Sigma) for 4 hours, and the formazan dye was solubilized by addition of acidic isopropanol. As a positive control, cells were incubated with normal culture medium. As a background control, all cells were lysed with Triton X100 before incubation with MTT reagent. The optical density (O.D.) of each well was quantified by using a microplate reader (Model 680 microplate reader, Bio-Rad).The test wavelength was 570nm and the reference was 630nm. All the experiments were performed in triplicate wells and repeated three times. The cell survival rate was expressed as % survival, which was calculated according to the following formula:
% Survival=(Experimental O.D.-Background O.D.) / (Positive control O.D - Background O.D.) x 100
Transfection of cultured cells
cDNAs encoding a full length human Livin cDNA and a full length human Survivin cDNA with an N-terminal myc-epitope-tag were amplified by PCR respectively. The PCR products were purified, sequenced, and cloned into pcDNA3/Amp mammalian expression vector (Invitrogen, Carlsbad, CA, USA). The resulting expression plasmids, pcDNA3-Myc-Livin and pcDNA3-Myc-Survivin, were used for the transfection. HEK293T cells (1x106) were transfected with the plasmids using Lipofectamine (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions. Forty-eight hours after the transfection, the cells were harvested.
Pull-down assay and Western blotting
The transfected HEK293 cells were washed in ice-cold PBS, homogenized in ice-cold CHAPS lysis buffer [20mM HEPES (pH8.0), 100mM NaCl, 0.5% CHAPS, protease inhibitor cocktail (Complete, Roche Diagnostics, Basel, Switzerland)] for 30 minutes, and clarified by centrifugation at 12,000 x g for 20 minutes at 4ºC. A part of the whole cell lysate was boiled for 5 minutes with SDS sample buffer and then separated by SDS-PAGE. The Smac7-Tat-His6 peptide and Smac7M-Tat-His6 peptide were each incubated with Probond Nickel-Chelating Resin (Invitrogen, Carlsbad, CA, USA) for 1 hour at 4ºC, followed by washing and incubation with the whole cell lysate for 24 hours at 4ºC. The beads were washed three times with ice-cold CHAPS lysis buffer, and the His6-tagged peptides were eluted by incubation with 200mM imidazole. The eluted His6-tagged peptides and proteins were boiled for 5 minutes with SDS sample buffer, separated by 12% SDS-PAGE, and then transferred electrophoretically to a polyvinylidene fluoride membrane (Immobilon-P, Millipore, Billerica, MA, USA). The membranes were incubated with blocking buffer (5% nonfat dry milk in PBS) at room temperature and then incubated for 60 minutes with the following antibodies: a mouse anti-Myc epitope tag monoclonal antibody (clone 9E10, American Type Culture Collection) and a mouse anti-His (5) antibody (Penta-His antibody, Qiagen, Valencia, CA, USA).After three washes with wash buffer (0.1% Tween 20, PBS), the membrane was reacted with a peroxidase-labeled secondary antibody (peroxidase-labeled goat anti-mouse IgG antibody; KPL, Gaithersburg, MD, USA) for 2 hours. Finally, the signal was visualized using an enhanced chemiluminescence detection system (Amersham Life Science, Arlington Heights, IL) according to the manufacturer’s protocol.
Statistical analysis
Data are presented as values of means ± standard deviation (SD) of at least three experiments. Statistical analysis was carried out with Student’s t-test. P values of less than 0.05 were considered statistically significant.
Results
Transduction of Smac7-Tat peptide into HeLa cells
The Smac7-Tat peptide labeled with FITC (1 µM) was added to the culture medium. Two hours later, the peptide was visualized using a fluorescence microscope. As shown in Figure1, the Smac7-Tat was successfully transduced into the cytosol of HeLa cells and partially transduced into the nucleus. Mean fluorescence intensity of cells with FITC-Smac7-Tat was 98.4 ± 7.7, whereas that without peptide was 59.7 ± 2.0.
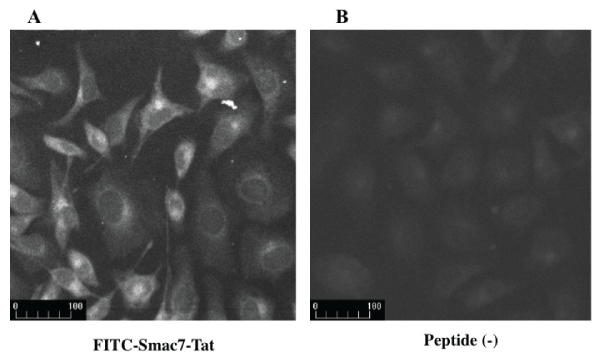
Figure 1: Transduction of Smac7-Tat peptide into HeLa cells
FITC-labeled peptide Smac7-Tat was added to the culture medium. Two hours later, the peptide was visualized by a fluorescence microscope. (A) Mean
fluorescence intensity of cells with FITC-Smac7-Tat was 98.4 ± 7.7. (B) Meanfluorescence intensity of cells without peptide was 59.7 ± 2.0.
View Figure 1
Cytotoxicity of Smac7-Tat peptide against HeLa cells
HeLa cells were incubated with various concentrations of Smac7-Tat peptide or Smac7M-Tat peptide for 24 hours (Figure 2A) or 72 hours (Figure 2B), and the cell viability was analyzed by MTT assay. Smac7-Tat reduced the cell viability significantly after incubation with 10 µM and 100 µM concentration of the peptide. However, Smac7M-Tat did not affect the survival rate even after incubation with the 100 µM concentration for 72 hours. These results were consistent with the previous reports that the N-terminal amino acid Ala of Smac7 was essential for the binding to IAP [15,16].
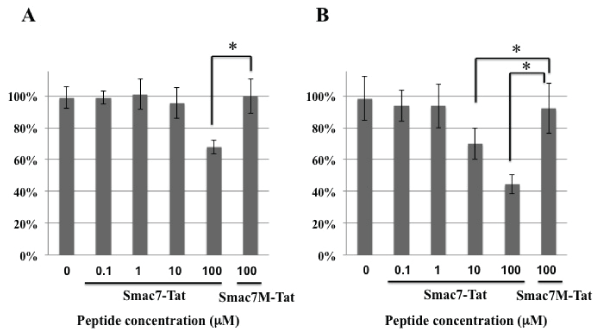
Figure 2: Cytotoxicity of Smac7-Tat peptide against HeLa cells
HeLa cells were incubated with the indicated concentrations of the Smac7-Tat peptide or Smac7M-Tat peptide for 24 hours (A) and 72 hours (B). The survival
rates were accessed by MTT assay and are expressed as % survival, which was calculated according to the formula described in the methods section. Data
are presented as values of eans ± standard deviations of at least three experiments. (*P<0.05)
View Figure 2
Cytotoxicity of Smac13-Tat peptide against HeLa cells, normal lymphocytes, and human embryonal kidney cells
HeLa cells, normal human peripheral blood lymphocytes (PBLs), and HEK293 cells were incubated with 100 µM Smac13, Smac7M-Tat, Smac7-Tat, Smac13M-Tat, or Smac13-Tat for 24 hours. Neither Smac13 nor Smac13M-Tat affected the survival rate of HeLa cells; however, Smac7-Tat and Smac13-Tat reduced the survival rate significantly (Figure 3A). Smac13-Tat had greater cytotoxicity against HeLa cells than Smac7-Tat. In contrast, neither Smac7-Tat nor Smac13-Tat affected the survival rate of normal PBLs (Figure 3B) or HEK293 cells (Figure 3C). These results indicated that Smac-Tat peptides might exert cytotoxicity against tumor cells, but not non-cancerous cells.
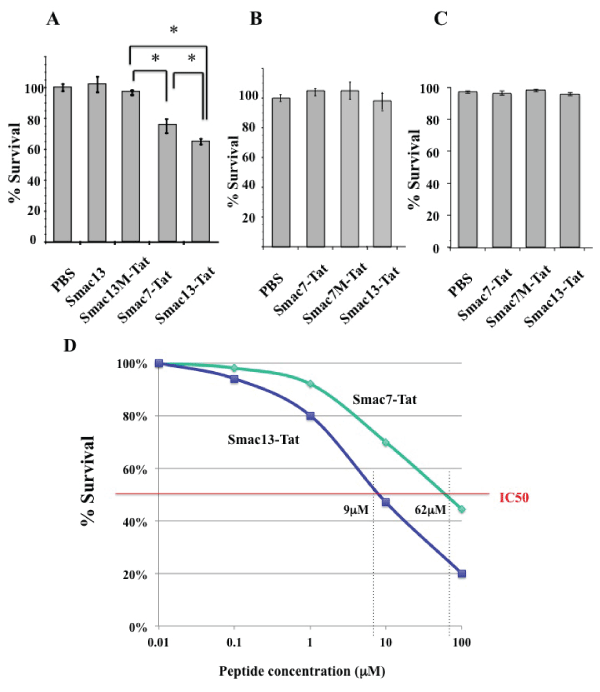
Figure 3: Cytotoxicity of Smac13-Tat peptide against HeLa cells and normal
lymphocytes
(A) HeLa cells were incubated with 100µM Smac13, Smac13M-Tat, Smac7-
Tat, or Smac13-Tat for 24 hours. (B) Normal human peripheral blood
lymphocytesand (C) HEK293 cells were incubated with 100µM Smac7-
Tat, Smac7M-Tat, or Smac13-Tat for 24 hours. The survival rates were
accessed by MTT assay and are expressed as % survival, which was
calculated according to the formula described in the methods section. Data
are presented as values of means ± standard deviations of at least three
experiments. (*P< 0.05) (D) HeLa cells were incubated with the indicated
concentrations of the Smac7-Tat peptide or Smac13-Tat peptide for 72 hours,
and the survival rates were analyzed by MTT assay. Data are presented
as mean values of triplicate experiments. IC50 of Smac13-Tat was 9µM,
whereas that of Smac7-Tat was 62µM.
View Figure 3
Then, we analyzed a half maximal inhibitory concentration (IC50) of Smac-Tat peptides in HeLa cells. HeLa cells were incubated with various concentrations of the Smac7-Tat peptide or Smac13-Tat peptide for 72 hours, and the survival rates were analyzed by MTT assay. IC50 of Smac13-Tat was 9 µM, whereas that of Smac7-Tat was 62 µM (Figure 3D)
Smac-Tat peptides increase chemosensitization of HeLa cells to VP-16-induced cell death
HeLa cells were incubated with or without VP-16 in the presence of 100 µM Smac-Tat. The survival rates were analyzed by MTT assay after incubation for 24 hours. The survival rate was decreased to ca. 70% in the presence of the Smac7-Tat peptide. After incubation with 250 µM VP-16 in the presence of 100 µM Smac7M-Tat or Smac13M-Tat, the survival rates were not changed; however, they were decreased to ca. 55% and 42% in the presence of Smac7-Tat and Smac13-Tat, respectively (Figure 4). The results indicated that Smac-Tat peptides increased the sensitivity of HeLa cells to the chemotherapeutic agent VP-16, and that Smac13-Tat had a more potent sensitizing effect than Smac7-Tat.
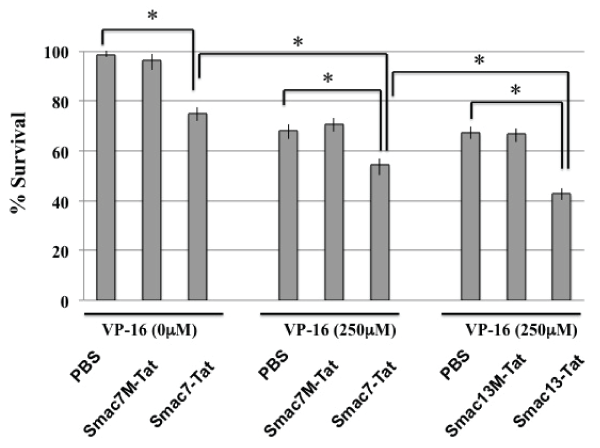
Figure 4: Chemosensitization of HeLa cells to VP-16-induced cell death by
Smac-Tat peptides
HeLa cells were incubated with or without 250µM VP-16 in the presence
of the indicated Smac-Tat peptide. The survival rates were analyzed by
MTT assay after incubation for 24 hours. The cell survival rate is expressed
as % survival, which was calculated according to the formula described in
the methods section. Data are presented as values of means ± standard
deviations of at least three experiments. (*P<0.05)
View Figure 4
Chemosensitization of HeLa cells to paclitaxel-induced cell death by Smac-Tat peptides
HeLa cells were incubated with 1 µM paclitaxel or 100 µM concentrations of Smac-Tat peptides for 24 hours and 40 hours, and the survival rates were analyzed by MTT assay. The survival rates were decreased to ca. 90% in the presence of 1 µM paclitaxel and ca. 70% in the presence of Smac13-Tat after incubation for 24 hours (Figure 5A). It was indicated that the HeLa cells used in the present study was resistant to paclitaxel-induced cell death, since it was previously reported that IC50 of paclitaxel in HeLa cells was 2.6nM [27]. In the presence of both 1 µM paclitaxel and 100 µM Smac13-Tat, the survival rate was decreased to ca. 45% after incubation for 24 hours, indicating that Smac13-Tat increased the cytotoxicity of paclitaxel against HeLa cells synergistically. Neither Smac13 nor Smac13M-Tat had such a chemosensitizing effect. Smac13-Tat had a more potent chemosensitizing effect than Smac7-Tat (Figure 5B).
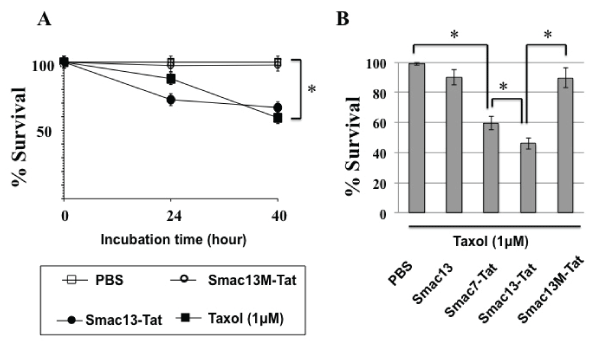
Figure 5: Chemosensitization of HeLa cells to paclitaxel-induced cell death
by Smac-Tat peptides
(A) HeLa cells were incubated with 1µM paclitaxel, 100µM Smac13-Tat or
100µM Smac13M-Tat for 24 hours and 40 hours, and then the survival rates
were analyzed by MTT assay. (B) HeLa cells were incubated with 1µM
paclitaxel in the presence of 100µM Smac13, Smac7-Tat, Smac13-Tat, or
Smac13M-Tat for 24 hours, and then the survival rates were analyzed by
MTT assay. The cell survival rate is expressed as % survival, which was
calculated according to the formula described in the methods section. Data
are presented as values of means ± standard deviations of at least three
experiments. (*P<0.05)
View Figure 5
Smac7-Tat peptide bound to Livin but not Survivin
To determine the target IAP molecules of the Smac-Tat peptides, we analyzed whether Livin and Survivin could bind to Smac7-Tat, since both of them were expressed in HeLa cells but not in normal PBLs. HEK293T cells were transfected with a Myc-tagged Livin or Myc-tagged Survivin fusion protein-expression vector, followed by harvesting of cell lysates. Nickel beads were preincubated with the Smac7-Tat-His6 or Smac7M-Tat-His6 peptide, and then incubated with the cell lysates. The His-tagged Smac-Tat peptides were eluted by imidazole and subjected to SDS-PAGE and Western blotting with an anti-myc tag antibody or anti-penta-His antibody. It was clearly demonstrated that Smac7-Tat could bind to Livin but not to Survivin (Figure 6). The Smac7M-Tat peptide could bind to neither Livin nor Survivin. These results indicated that Smac-Tat peptides might exert cytotoxic and chemosensitizing effects via interaction with Livin in HeLa cells.
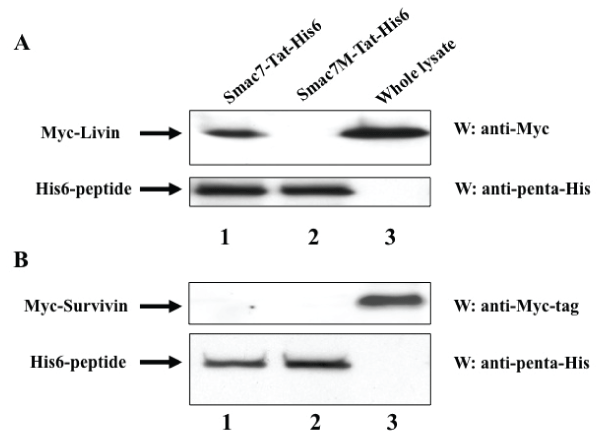
Figure 6: Smac7-Tat peptide binds to Livin but not Survivin
(A) HEK293T cells were transfected with a pcDNA3-Myc-Livin expression
vector, followed by harvesting of cell lysates. (B) HEK293T cells were
transfected with a pcDNA3-Myc-Survivin expression vector, followed by
harvesting of cell lysates. Nickel beads were preincubated with Smac7-Tat-
His6 or Smac7M-Tat-His6, and then incubated with the cell lysates. Histagged
peptides were eluted using 200 mM imidazole. The eluted peptides/
proteins (lanes 1 and 2) and whole cell lysate (lane 3) were subjected to
SDS-PAGE and Western blotting with an anti-Myc-tag antibody or anti-penta-
His antibody.
View Figure 6
Discussion
Smac is aproapoptotic mitochondrial factor and functions as an endogenous IAP inhibitor [21]. It binds to the BIR domain in IAP family proteins through the N-terminal AVPI tetrapeptide domain (amino acid residues 54-57) after the proteolytic cleavage in the N-terminal mitochondrial import signal sequence (amino acid residues 1-53) [15,22,28]. The structure of the tetrapeptide domain is conserved not only among mammalian Smac homologues, but also among the insect IAP binding proteins Grim, Reaper, and Hid [21]. Mutation of the very first amino acid Ala to Met leads to loss of the interaction with IAPs [15,22,23] . Therefore, we used Smac7M-Tat and Smac13M-Tat as negative control peptides. It has been reported that the first 7 amino acid sequence (amino acid residues 54-60), Smac7 is sufficient for the binding and suppression of the anti-apoptotic function of IAPs [16,22]. Several studies have shown that transduction of Smac7 or its mimetics into cells renders tumor cells sensitive to proapoptotic stimuli such as chemotherapeutic agents and irradiation [29-31]. Cell-penetrating peptides such as antennapedia peptide and HIV-Tat peptide have been used to efficiently transduce the Smac7 peptide into the cytosol [17,19]. In this study, the HIV-Tat peptide was used for the transduction. Since the 13 amino acid N-terminal sequence (amino acid residues 54-66), Smac13 is conserved among mammalian Smac homologues [15,21], we compared the cytotoxicities of Smac7-Tat and Smac13-Tat against human tumor cells and normal lymphocytes. It was clearly demonstrated that Smac13-Tat had higher cytotoxicity and a greater ability to sensitize HeLa cells to chemotherapeutic agents, VP-16 and paclitaxel. In contrast, Smac13-Tat exerted no cytotoxic effect against normal PBLs. These results suggested that Smac-Tat peptides might preferentially target IAP molecules that are expressed in tumor cells but not in normal lymphocytes. Among the IAP family proteins, expression of Survivin, Livin and ILP-2 is very limited in normal adult tissues. Since Survivin and Livin are expressed in HeLa cells, we examined whether Smac7-Tat could bind to these IAP family proteins. Our data showed that Smac7-Tat could bind to Livin but not to Survivin. The results were inconsistent with previous reports that Smac could bind and suppress various IAPs, including Survivin [29]. It is speculated that each BIR domain of IAP has distinct affinity to the Smac7 peptide, and that a larger peptide might be necessary for binding to Survivin. Actually, amino acid sequence alignment studies revealed that the homology of the BIR domain of Livin to those of XIAP-BIR3 and cIAP2-BIR3 was 50.0% and 51.5%, respectively, whereas that to the BIR domain of Survivin was 39.7% [32].
Our study suggests that Smac13-Tat might be suitable for use in molecular targeted therapy for Livin-positive cancer. A number of studies have shown that Livin is over expressed in various human solid cancers such as cervical cancer, lung cancer, colon cancer, kidney cancer and malignant melanoma, and its expression is correlated with a worse prognosis of cancer patients [5,8,10,11]. Most importantly, Livin-expressing cancer cells might be dependent on the function of Livin for cell survival, considering that inhibition of its expression or function leads to cell death in various cancer cells [11,12]. Chemoresistant cancer cells express increased levels of Livin, which suppresses the activation of caspases, thereby leading to decreased sensitivity to chemotherapeutic agents [12].
In summary, we have shown that (1) Smac7/13-Tat could sensitize HeLa cells to chemotherapeutic drugs, (2) Smac13-Tat is much better than Smac7-Tat in the chemosensitization of HeLa cells, and (3) Livin instead of Survivin may mediate the effects of Smac7.Our study suggests that Smac13-Tat and its peptidomimetics might serve as potent molecular targeted therapy agents for chemoresistant cervical cancer.
Acknowledgements
The present study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, program for developing the supporting system for upgrading education and research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
-
Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC (1997) The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 16: 6914-6925.
-
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, et al. (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17: 2215-2223.
-
Saleem M, Qadir MI, Perveen N, Ahmad B, Saleem U, et al. (2013) Inhibitors of apoptotic proteins: new targets for anticancer therapy. Chem Biol Drug Des 82: 243-251.
-
Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, et al. (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58: 5315-5320.
-
Kasof GM, Gomes BC (2001) Livin, a novel inhibitor of apoptosis protein family member. J Biol Chem 276: 3238-3246.
-
Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, et al. (2000) Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res 91: 1204-1209.
-
Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, et al. (2003) Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood 102: 571-576.
-
Hirohashi Y, Torigoe T, Maeda A, Nabeta Y, Kamiguchi K, et al. (2002) An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res 8: 1731-1739.
-
Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, et al. (2001) Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer 95: 92-95.
-
Myung DS, Park YL, Chung CY, Park HC, Kim JS, et al. (2013) Expression of Livin in colorectal cancer and its relationship to tumor cell behavior and prognosis. PLoS One 8: e73262.
-
Liu B, Han M, Wen JK, Wang L (2007) Livin/ML-IAP as a new target for cancer treatment. Cancer Lett 250: 168-176.
-
Wang R, Lin F, Wang X, Gao P, Dong K, et al. (2008) Silencing Livin gene expression to inhibit proliferation and enhance chemosensitivity in tumor cells. Cancer Gene Ther 15: 402-412.
-
Hariu H, Hirohashi Y, Torigoe T, Asanuma H, Hariu M, et al. (2005) Aberrant expression and potency as a cancer immunotherapy target of inhibitor of apoptosis protein family, Livin/ML-IAP in lung cancer. Clin Cancer Res 11: 1000-1009.
-
Vaux DL, Silke J (2003) Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun 304: 499-504.
-
Wu G, Chai J, Suber TL, Wu JW, Du C, et al. (2000) Structural basis of IAP recognition by Smac/DIABLO. Nature 408: 1008-1012.
-
Chai J, Du C, Wu JW, Kyin S, Wang X, et al. (2000) Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406: 855-862.
-
Du LQ, Wang Y, Xu C, Cao J, Wang Q, et al. (2013) Radiation-sensitising effects of antennapedia proteins (ANTP)-SmacN7 on tumour cells. Int J Mol Sci 14: 24087-24096.
-
Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, et al. (2003) Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res 63: 831-837.
-
Chen F, Xu C, Du L, Wang Y, Cao J, et al. (2013) Tat-SmacN7 induces radiosensitization in cancer cells through the activation of caspases and induction of apoptosis. Int J Oncol 42: 985-992.
-
Kim D, Jeon C, Kim JH, Kim MS, Yoon CH, et al. (2006) Cytoplasmic transduction peptide (CTP): new approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res 312: 1277-1288.
-
Silke J, Verhagen AM, Ekert PG, Vaux DL (2000) Sequence as well as functional similarity for DIABLO/Smac and Grim, Reaper and Hid? Cell Death Differ 7: 1275.
-
Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, et al. (2000) Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem 275: 36152-36157.
-
Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, et al. (2000) Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature 408: 1004-1008.
-
Vivès E, Brodin P, Lebleu B (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272: 16010-16017.
-
Morris MC, Depollier J, Mery J, Heitz F, Divita G (2001) A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 19: 1173-1176.
-
Console S, Marty C, García-Echeverría C, Schwendener R, Ballmer-Hofer K (2003) Antennapedia and HIV transactivator of transcription (TAT) "protein transduction domains" promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem 278: 35109-35114.
-
Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, et al. (1993) Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer 68: 1104-1109.
-
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33-42.
-
Guo F, Nimmanapalli R, Paranawithana S, Wittman S, Griffin D, et al. (2002) Ectopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood 99: 3419-3426.
-
Yang J, McEachern D, Li W, Davis MA, Li H, et al. (2011) Radiosensitization of head and neck squamous cell carcinoma by a SMAC-mimetic compound, SM-164, requires activation of caspases. Mol Cancer Ther 10: 658-669.
-
Zhou W, Shi J, Niu Z, Luo H, Tian H, et al. (2013) SmacN7 enhances the sensitivity of pancreatic cancer cells to tumor necrosis factor-related apoptosis-inducing ligand or gemcitabine. Oncol Lett 5: 1760-1764.
-
Wang L, Zhang Q, Liu B, Han M, Shan B (2008) Challenge and promise: roles for Livin in progression and therapy of cancer. Mol Cancer Ther 7: 3661-3669.





