International Journal of Blood Research and Disorders
Significance of sCD86 Levels in Acute Myelogenous Leukemia Patients: An Egyptian Study
Naglaa M Hassan1, Nevin M Al-azhary1,2* and Marwa M Hussein3
1Department of Clinical Pathology, National Cancer Institute, Cairo University, Cairo, Egypt
2Department of Biochemistry and Molecular Medicine, College of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia
3Department of Medical Oncology, National Cancer Institute, Cairo University, Cairo, Egypt
*Corresponding author:
Nevin Mohamed Fathy Al azhary, Assistant professor of clinical pathology, National Cancer Institute, Cairo University, Cairo, Egypt, Tel: 00966555276515, E-mail: nevin_elazhary@hotmail.com
Int J Blood Res Disord, IJBRD-3-021, (Volume 3, Issue 1), Research Article; ISSN: 2469-5696
Received: April 16, 2016 | Accepted: May 25, 2016 | Published: May 28, 2016
Citation: Hassan NM, Al-azhary NM, Hussein MM (2016) Significance of sCD86 Levels in Acute Myelogenous Leukemia Patients: An Egyptian Study. Int J Blood Res Disord 3:021. 10.23937/2469-5696/1410021
Copyright: © 2016 Hassan NM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The prognostic significance of sCD86 in patients with hematologic malignancies is unclear. We evaluated sCD86 levels in 63 newly diagnosed AML and 16 controls by enzyme-linked immunosorbent assay and then analyzed how its levels and various clinical parameters related to overall survival. Levels of sCD86 in patients were high (1.22 ± 1.34) compared with the controls (0.51 ± 0.23) but were not significant (p = 0.096). The patients' outcome and whether they achieved complete remission and if they were sCD86 positive or negative didn't have any significance (p = 0.1). High levels of sCD86 were detected in patients with hypercellular marrow (with a high percentage of bone marrow blasts), FLT-3 mutated type, and in FAB M4-M5. The overall survival among sCD86-positive and sCD86-negative patients was not significant (p = 0.16). The overall survival of patients regarding clinical parameters showed no significance except for FLT-3 type (p = 0.001).
Aim of the Study: The aim of the study is to assess the level of soluble CD86 (sCD86) in patients with de novo AML and to compare them with a normal control group to determine any possible role with prognosis and clinical outcome, as the significance of sCD86 in hematologic malignancies is still controversial.
Keywords
AML, sCD86, ELISA, Prognosis
Introduction
CD4+ T helper cells (TH) cell activation is initiated by the interaction of the T-cell receptor (TCR) CD3 complex with antigen presenting cell (APC) through the antigenic peptide bound to the MHC class II molecule on its surface [1].
Naïve T lymphocytes require two diverse signals from APCs to be a functional cell. The first one is the previously mentioned interaction between it and APC, which confers specificity. A second signal can be provided by APC-borne ligands for the CD28 and cytotoxic lymphocyte-associated antigen-4 (CTLA-4) receptors on T cells [2]. The B7 molecules CD80 and CD86 are expressed predominantly by activated APCs, and they generate, following binding to their T-cell ligands (CD28, CTLA-4), bi-directional signals that are critical in the regulation of antitumor responses. The B7:CD28 interaction enhances and the B7:CTLA-4 inhibits both APC and effector cell function [3]. CTLA-4, with its ligands interaction, is stronger than the interaction of CD28 with their ligands [4]. Despite having the same ligands, CD80 and CD86 appear to be involved in different mechanisms: CD80 can be more potent than CD86 in inducing an antitumoral response, while CD86 preferentially induces the production of a helper (Th) 2 response.
The transcript for soluble CD86 (sCD86) is expressed in many cells as normal monocytes, dendritic cells, as well as some leukemic cells [5]. The antitumor responses of membrane B7 (mB7) expression on APC increases the hypothesis of tumor development out of immune responses and may occur in part from low expression of B7 by the malignant cell population. But many studies have demonstrated that expression of membrane CD86 (mCD86) by the malignant cells is associated with poor prognosis [3]. Soluble forms of membrane molecules are typically released as either the product of specific mRNA or as a result from cleavage of the cognate membrane form. Both normal and leukemic cells can express mCD86 and sCD86 transcript. The release of soluble forms of membrane molecules has an immunoregulatory role and provides a strong way for leukocytes to modulate their biologic effects according to membrane counterparts. However, the mechanisms for the production of sCTLA-4, sCD28, sCD80, and sCD86 and their correlation with hematological malignancy activity have not been well elucidated [6].
Many antigens on the leukocyte cell surface are detached as soluble forms and have different biological effects, such as agonistic, antagonistic, or independent function, depending of if they are membrane bound. Fifteen alternately spliced transcript encodings for soluble forms of porcine CD80, canine CD80 and canine CD86 have also been reported [7].
It is known that many tumor cells release soluble molecules that can inhibit immune responses and that sCD86 release represents a mechanism by which tumor cells escape anti-tumor responses [8,9]. However, many factors influence tumor immunogenicity, and tumor cell expression of B7 does not in itself confer immunogenicity; many hematological malignancies express B7 constitutively, and its expression was associated with a low therapeutic response and poor prognosis [7].
Around 20% to 50% of patients with AML are primarily resistant to induction chemotherapy; it has previously been shown that resistance to the first cycle of induction chemotherapy is an independent prognostic factor [10,11]. Treatment failure remains the main clinical challenge for these patients.
Leukemic cells in acute myeloid leukemia from a considerable number of patients expressed B7.2, but there were incompatible results regarding the correlation with bad prognosis and outcome [12,13]. The tumor cells may actively suppress an immune response through a number of mechanisms, including direct tolerization of tumor-reactive T cells, suppression of tumor-reactive T, ignorance of tumor as a result of spatial separation of T and tumor cells, and tolerization of host T cells by cross-presentation of tumor-derived antigens [12].
Materials and Methods
This is a matched case-control study, which included 63 newly diagnosed AML patients that were presented to the Adult Oncology Department of the National Cancer Institute (NCI), Cairo University, between July 2013 and January 2015. Clinical, morphological, cytochemical, and flow cytometric analyses were done for proper diagnosis. Cytogenetic analysis and FLT-3 detection are routine investigations done in NCI for their prognostic value. Sixteen healthy volunteers were also included as a control group. The participants of the two groups had no significant differences based on age and sex distribution. All subjects included in the study were aware of their participation, were knowledgeable about the study, and had willingly signed a consent form. The study protocol was approved by the Medical Ethics Committee of the National Cancer Institute.
Blood sampling
Three milliliters of peripheral venous blood were withdrawn from every participant under completely aseptic conditions, and after separation, the plasma was stored at 70°C. Assessment of serum levels of sCD86 was achieved using Human sCD86 ELISA Kit Sunlong Biotech Cat No SL1600Hu, which implies a Sandwich ELISA technique in which the micro-ELISA strip plate is precoated with an antibody specific to sCD86. The optical density of the developing color is measured spectrophotometrically at wavelength 450 nm. The optical density value is proportional to the concentration of sCD86.
Statistical methods
Data management and analysis were performed using the Statistical Package for Social Sciences (SPSS), vs. 21. Numerical data were summarized using means and standard deviations or medians and ranges, as appropriate. Categorical data were summarized as numbers and percentages. Numerical data were explored for normality using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. Exploration of data revealed that the collected values were not normally distributed. Comparisons between the two groups were done by the Mann-Whitney test. Overall survival time was measured from the time of diagnosis until time of death or loss to follow-up. It was estimated using the methods of Kaplan and Meier. Differences between survival curves were assessed for statistical significance with the log-rank test. All p values are two-sided: p < 0.05 are considered significant.
Results
In our study we assessed levels of sCD86 in 63 de novo acute myeloid leukemia patients compared with 16 volunteers in the control group to determine if there is any correlation with prognosis, outcome, and overall survival.
The AML patients included were 29 males (46%) and 34 females (54%), and their ages ranged from 18 to 68 years with a mean value of 38.4 ± 13.6 years. The control group included 16 healthy adults, specifically 9 males (56.3%) and 7 females (34.8%), and their ages ranged from 29 to 58 years with a mean of 36.88 ± 7.9 years.
Table 1 represents laboratory investigations of the patient group, which included total leucocytic count, HB, peripheral, and BM blasts.
![]()
Table 1: Lab investigation of the patients group.
View Table 1
Cytogenetic characterizations of the cases were as follow: 35 cases (55.5%) had normal karyotyping, and numerical abnormality was seen in 17 cases (48.5%). Finally, 11 cases (17.4%) had structural abnormality.
Regarding the distribution of FAB subtypes among patients, 3 patients were M0 (4.8%), 24 patients were M2 (38%), 13 patients were M1 (20.7%), 6 patients were M3 (9.5%), 12 patients were M4 (19%), 2 patients were M5 (3.2%), 2 patients were M7 (3.2%), and one case was biphenotypic (1.6%).
The clinical outcome after the first cycle of chemotherapy was as follow: 38 patients achieved CR (60.3%), 22 patients did not achieve CR (34.9%), and about 3 patients were lost to follow-up (4.7%).
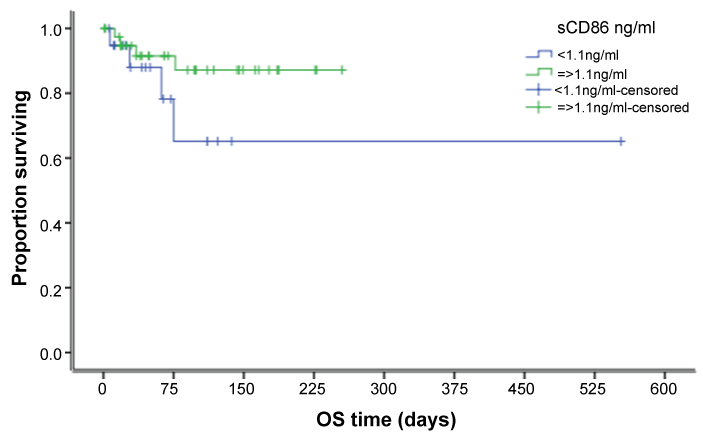
Soluble CD86 was detected in the plasma of all normal controls and patients included in the study. Levels of sCD86 in the patients ranged from 0.20 to 7.6 ng/mL with a mean of 1.22 ± 1.34. In the control group, levels of sCD86 ranged from 0.28 to 1.1 ng/mL with a mean of 0.51 ± 0.23. There was no significant difference between the levels detected in the normal control and patient groups p = 0.096. (Table 2). About 54% had sCD86 levels higher than the levels detected in normal donors (1.1 ng/mL), and about 3.1% of patients had levels greater than 5 ng/mL.
![]()
Table 2: p value = 0.096.
View Table 2
The patients were divided into two groups based on their plasma levels of sCD86. The cut-off level was set at1.1 ng/mL, which represents a value equal to the upper level measured in the control group. Patients with levels lower than 1.1 ng/mL were defined as the low group, and patients with levels equal to or above 1.1 ng/mL were defined as the high group.
Regarding bone marrow cellularity in sCD86 high group, 17 cases (26.9%) were hypercellular, 8 cases (12.6%) were extremely hypercellular, and 11 cases (17.4%) were normocellular. In the sCD86 low group, 12 cases (19%) were hypercellular, 5 cases (7.9%) were extremely hypercellular, and 10 cases (15.8%) were normocellular.
In regard to the positivity for soluble CD86, 34 patients (53.9%) were high and 29 patients (46%) were low. The outcome in relation to sCD86 levels was assessed with the following results: in sCD86-high patients, 21 cases achieved complete remission, 12 cases did not, and one case was lost to follow-up. Also among sCD86 low patients, 14 cases achieved complete remission, 13 cases did not achieve complete remission, and 2 cases were lost to follow-up. This showed no significance (p = 0.1).
![]()
Table 3: Multivariate Analysis of the Correlation between Characteristics and Survival in Patients with Acute Myeloid Leukemia.
View Table 3
According to the FAB classification, comparison of sCD86 levels within the different AML subtypes demonstrated that 12 out of 14 patients with monocytic morphology (M4-M5), 66.6% were having elevated sCD86 levels, while in other FAB subtypes, about 36.5% were having levels elevated above normal control.
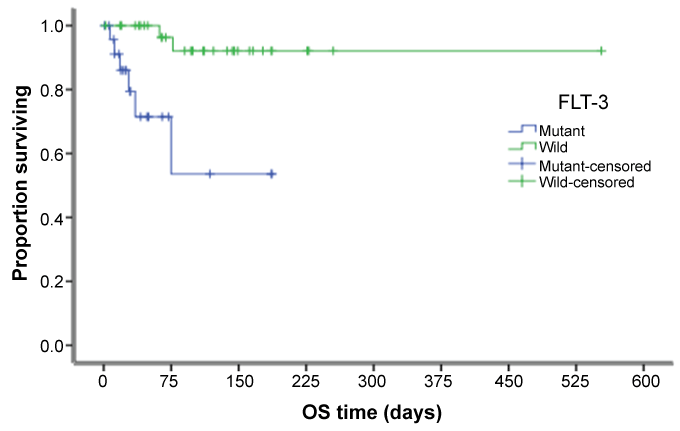
Table 3 represents the overall survival of patients regarding many factors, which showed no significant value except for FLT-3 status, whether mutant or wild, that showed a high significant value (p = 0.001). Figure 1 and figure 2 shows overall survival regarding level of sCD86 and FLT-3 status respectively.
Discussion
For a better understanding of the molecular, cytogenetic and immunological mechanisms of acute myeloid leukemia (AML) and its poor chemotherapy outcome, the detection of novel diagnostic and prognostic markers is vital because we can use them as a guide to develop new targeted chemotherapies or immunotherapeutic agents. Elevated levels of soluble CD80 and CD86 in some leukemia patients have been demonstrated. However, the mechanisms for producing sCTLA-4, sCD28, sCD80, and sCD86 and their association with hematological malignancy have not been well elucidated.
The aim of this study is to evaluate the level of sCD86 in a group of patients with AML, comparing them with a normal group, and to correlate their levels with the hematological findings, response to therapy, and overall survival. A wider range of levels was observed in patients with newly diagnosed AML (0.20 to7.6 ng/mL), but we found no significant statistical difference between sCD86 expression levels in patients versus their controls (p = 0.096). These results agree with those of Barry D. Hock et al. [14], who found no difference between patients and controls (p = 0.93). In contrast, our results didn't match a study conducted by Nahla Hamed et al. [1], who found a significant difference between cases and controls (p = 0.001).
Our study showed that the majority of cases had high levels of sCD86 above normal values (53%), which is similar to Magda Assem et al. [15], who reported 53.3% positivity, and with Whiteway et al. [16], who reported 57% positivity. Otherwise, our results disagree with Barry D. Hock et al. [14], who reported only 25% with high levels.
We found no significant association between the expression of sCD86 and the response to therapy or the outcome after the first cycle of therapy (p = 0.1); this was in agreement with Nahla Hamed et al. [1], who found that patients achieving complete remission were 27.3% in sCD86-positive cases and 42% in sCD86-negative cases. In contrast, Barry D. Hock et al. [14], found a significant statistical difference in response to therapy (p = 0.021).
Our study revealed that sCD86-high AML patients had a significantly higher bone marrow cellularity (26.9% of cases had hypercellular marrow and 12.6% had extremely hypercellular marrow) compared with the sCD86 low patients, where 19% of cases had hypercellular marrow and 7.9% of cases had extremely hypercellular marrow. This was similar to Tamura et al. [17], who reported that sCD86-positive AML patients had a significantly higher leukocytic count compared with the sCD86-negative patients.
Our results regarding FLT-3 analysis in sCD86-high AML patients showed that a mutant type was reported in 18 cases and a wild type in 16 cases. In sCD86-low patients, 10 cases were mutant and 19 cases wild; therefore, the mutant type increased in sCD86-high AML patients but was not statistically significant, which may be due to decreased sample size, as FLT3 is considered one of the bad prognostic markers in AML.
We observed that patients with elevated sCD86 levels predominantly found in FAB M4-M5 subtypes, which agrees with Nahla Hamed et al. [1] and Barry D. Hock et al. [14].
In our study, the overall survival (OS) among sCD86-high and sCD86-low patients was not significant (p = 0.16). OS of patients regarding BM cellularity, FAB classification, cytogenetics, and BM blasts also showed no significant difference, except for FLT-3 type (p = 0.001). These results were consistent with those of H. Tamura [17], who did not find sCD86 expression to be an independent prognostic factor.
Our results agree with Barry D. Hock et al. [6], who studied the role of both sCD86 and CD80 in AML patients and found that sCD86 had no role in prognosis. They found no difference between patients and controls while sCD80 is functional, and its comparison with sCD86 levels demonstrated that sCD80 was independently elevated in 39% of patients and has a bad prognostic role.
Barry D. Hock et al. [6,7] and Magda Assem et al. [15] found that elevated sCD86 levels have been reported as a marker of poor prognosis in acute myeloid leukemia. They suggest that CD86 on leukemic cells intensify the production of IL-4 and IL-10, which inhibits tumor-specific TH1 and CTL activation and differentiation. They suggest that sCD86-release provides a mechanism by which malignant cells inhibit the immune response.
We conclude that there is no statistical significance in sCD86 regarding outcome and overall survival; however, high levels of sCD86 were detected in patients with hypercellular marrow (a high percentage of bone marrow blasts), FLT3 mutated type, and in FAB M4-M5. The previous observation indicates a possible prognostic role for sCD86 positive in AML patients. A larger-scale study is recommended to better illustrate the findings.
References
-
Hamed N, Farahat N, El Sorady M, Nafee DA, Barakat S (2011) Clinical significance of sCD86 levels in patients with acute myelogenous leukemia. Alexandria Journal of Medicine 47: 25-30.
-
Shi HZ, Xie ZF, Deng JM, Chen YQ, Xiao CQ (2004) Soluble CD86 protein in serum samples of patients with asthma. Thorax 59: 870-875.
-
Hock BD, Drayson M, Patton WN, Taylor K, Kerr L, et al. (2006) Circulating levels and clinical significance of soluble CD86 in myeloma patients. Br J Haematol 133: 165-172.
-
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23: 515-548.
-
Mansour A, Elkhodary T, Darwish A, Mabed M (2013) Increased expression of costimulatory molecules CD86 and sCTLA-4 in patients with acute lymphoblastic leukemia. Leukemia & Lymphoma 55: 2120-2124.
-
Hock BD, Starling GC, Patton WN, Salm N, Bond K, et al. (2004) Identification of a circulating soluble form of CD80: levels in patients with hematological malignancies. Leuk Lymphoma 45: 2111-2118.
-
Hock BD, Patton WN, Budhia S, Mannari D, Roberts P, et al. (2002) Human plasma contains a soluble form of CD86 which is present at elevated levels in some leukaemia patients. Leukemia 16: 865-873.
-
Fló J, Tisminetzky S, Baralle F (2001) Codelivery of DNA coding for the soluble form of CD86 results in the down-regulation of the immune response to DNA vaccines. Cell Immunol 209: 120-131.
-
Runyon K, Lee K, Zuberek K, Collins M, Leonard JP, Dunussi-Joannopoulos K (2001) The combination of chemotherapy and systemic immunotherapy with soluble B7-immunoglobulin G leads to cure of murine leukemia and lymphoma and demonstration of tumor-specific memory responses. Blood 97: 2420-2426.
-
Alazhary NM, Shafik RE, Shafik HE, Kamel MM (2015) Prognostic Value of a CYP2B6 Gene Polymorphism in Patients with Acute Myeloid Leukemia. Asian Pac J Cancer Prev 16: 4583-4587.
-
Su L, Li X, Gao SJ, Yu P, Liu XL, e t al. (2014) Cytogenetic and genetic mutation features of de novo acute myeloid leukemia in elderly Chinese patients. Asian Pac J Cancer Prev 1: 895-898.
-
Alarcón B, Gil D, Delgado P, Schamel WW (2003) Initiation of TCR signaling: regulation within CD3 dimers. Immunol Rev 191: 38-46.
-
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23: 515-548.
-
Hock BD, McKenzie JL, Patton WN, Haring LF, Yang Y, et al. (2003) The clinical significance of soluble CD86 levels in patients with acute myeloid leukemia and myelodysplastic syndrome. Cancer 98: 1681-1688.
-
Assem M, Raslan HN, Salem S, Abd El Aziz RS, El Said AM (2013) Combined CD86 Expression & Increase in Soluble Vascular Endothelial Growth Factor Confers Bad Prognosis in Adult Acute Myeloid Leukemia. Life Science Journal 10.
-
Whiteway A, Corbett T, Anderson R, Macdonald I, Prentice HG (2003) Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol 120: 442-451.
-
Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, et al. (2005) Expression of functional B7-H2 and B7.2 co-stimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res 11: 5708-5717.