International Journal of Blood Research and Disorders
The Use of Diffusion Weighted Imaging in the Diagnosis of Intraocular Relapse of Acute Lymphoblastic Leukemia: A Case Report
Kee Kiat Yeo1*, Jonathan G. Murnick2, Marijean M. Miller3, and Anne L. Angiolillo1,4
1Department of Pediatrics, Children's National Medical Center, USA
2Department of Diagnostic Imaging and Radiology, Children's National Medical Center, USA
3Department of Ophthalmology, Children's National Medical Center, USA
4Division of Oncology, Children's National Medical Center, USA
*Corresponding author:
Kee Kiat Yeo, MD, Department of Pediatrics, Children's National Medical Center, Washington DC 20010, USA, Tel: 202 476 5000, E-mail: aarony2k@gmail.com
Int J Blood Res Disord, IJBRD-2-009, (Volume 2, Issue 1), Case Report; ISSN: 2469-5696
Received: January 07, 2015 | Accepted: February 20, 2015 | Published: February 23, 2015
Citation: Yeo KK, Murnick JG, Miller MM, Angiolillo AL (2015) The Use of Diffusion Weighted Imaging in the Diagnosis of Intraocular Relapse of Acute Lymphoblastic Leukemia: A Case Report. Int J Blood Res Disord 2:009. 10.23937/2469-5696/1410009
Copyright: © 2015 Yeo KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Intraocular relapse of Acute Lymphoblastic Leukemia (ALL) is rare. Prompt diagnosis and treatment can minimize vision loss. We report a case of a young girl diagnosed with pre-B ALL who was initially treated successfully with chemotherapy. She presented 2˝ years after completion of therapy with unilateral vision changes. Using Magnetic Resonance Imaging (MRI) with diffusion Weighted Imaging (DWI), we localized the intraocular lesions and suspected a malignant lesion. Further studies confirmed Central Nervous System (CNS) relapse of ALL. This report supports the use of DWI in differentiating benign and malignant lesions of the globe, and in our case, intraocular relapse of ALL.
Keywords
Diffusion weighted imaging, Intraocular lesions, Acute lymphoblastic leukemia, Relapse, Malignant
Introduction
Leukemia is the most common malignancy in children, with ALL being the most commonly encountered in children. Approximately 2500-3500 new cases of ALL are diagnosed every year in the United States. With intensive multiagent chemotherapy, the overall survival rates have improved to approximately 80-90% [1]. However, the risk of relapse is significant, occurring in approximately 20%-25% of children. CNS relapse occurs in approximately 2-10% of patients, with isolated orbital relapse being much rarer. When ocular involvement is detected, early treatment is necessary as rapid progression can lead to vision loss or may require surgical enucleation [2]. Conventional Computed Tomography scan and MRI have both been useful in the diagnosis of intraocular lesions; however, neither is particularly useful in differentiating between benign and malignant lesions. Diffusion weighted magnetic resonance imaging (DWI) is a method of imaging that allows for the mapping of the diffusion process of molecules, primarily water [3]. This technique is useful for early diagnosis of acute ischemic injury to the brain, as well as identification of densely cellular tumors such as lymphoma. DWI has been used to help differentiate malignant from nonmalignant extra-ocular orbital lesions, including lymphoma [4-6]. We present a case of a 9 year old girl with a history of ALL who presents with vision changes in her right eye, found to have CNS relapse with the assistance of DWI.
Case History
A 4 year old girl presented to her primary care physician with complains of gum bleeding and recurrent low grade fevers. Her physical exam was notable for pallor and a complete blood count showed significant pancytopenia. Bone marrow aspiration was performed and revealed pre B acute lymphoblastic leukemia; CNS negative disease. She was treated on the Children’s Oncology Group (COG) standard risk protocol, AALL0331. Two months into her treatment, she complained of visual changes and was found to have preretinal hemorrhage in both eyes possibly secondary to thrombocytopenia and/or asparaginase. She was treated with fresh frozen plasma and subsequently, the right eye blood cleared but after two months, the left eye required vitrectomy to remove a collection of premacular blood to prevent complete vision loss from amblyopia. A small retinal hole occurred during surgery and was stabilized with laser. After amblyopia treatment and strabismus surgery on the left eye, the vision was 20/20 in each eye with high grade stereoacuity measuring 40 seconds of arc. After 2 years of multi agent chemotherapy and intrathecal therapy, she was in remission.
Two and a half years after completion of her therapy and with regular eye follow-up examinations, it was determined that part of the side vision was missing in the right eye. On examination, vision was counting fingers at 2 feet in the right eye with afferent pupillary defect and 20/20 in the left. Full ophthalmologic exam revealed concerns for a mass lesion with associated retinal detachment in the right eye.
MRI of the orbit and brain with and without gadolinium contrast and with diffusion weighted imaging was obtained revealing retinal detachment with an underlying mass in her right eye. Given her history of leukemia as well as previous retinal hemorrhage, both of these possibilities were considered in the differential diagnosis. Other etiology considered in the differential included orbital cellulitis, dermoid cyst and other oncological process. Precontrast T1 weighted images demonstrated a mass in the posterior globe, which was uniformly T1-isointense to cerebral gray matter. However, post-gadolinium T1 weighted images and T2 weighted images demonstrated two components to the mass: an anterior T2-hyperintense, nonenhancing component, and a posterior T2-hypointense, enhancing component. Imaging characteristics of the anterior component were consistent with subretinal hematoma or proteinaceous exudate. Uniform enhancement identified the posterior component as solid tissue, rather than bland hematoma. The posterior component of the lesion demonstrated uniform restricted diffusion (ADC=6.3×10e-4 mm2/s), suggesting dense cellularity as in a leukemic infiltrate (Figure A1, A2, B1, B2).
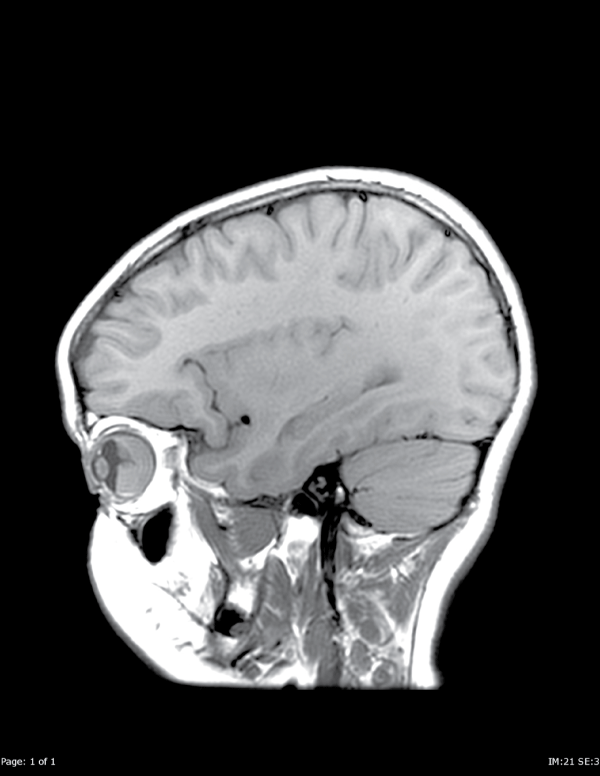
Figure A1: Sagittal T1 pre-contrast images demonstrate a mass in the posterior right globe.
View Figure 1
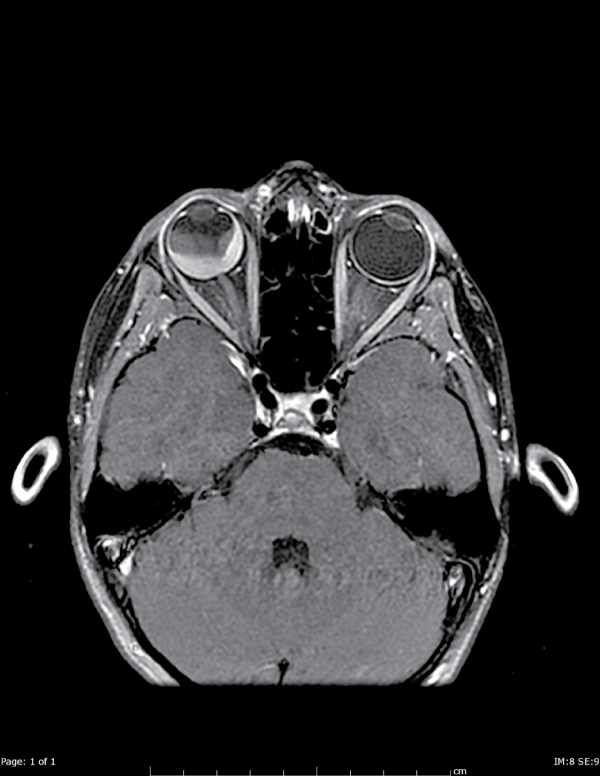
Figure A2: Axial T1 postcontrast images show that the mass is comprised of two components. The posterior component enhances with IV contrast,
inconsistent with a bland hematoma.
View Figure 2
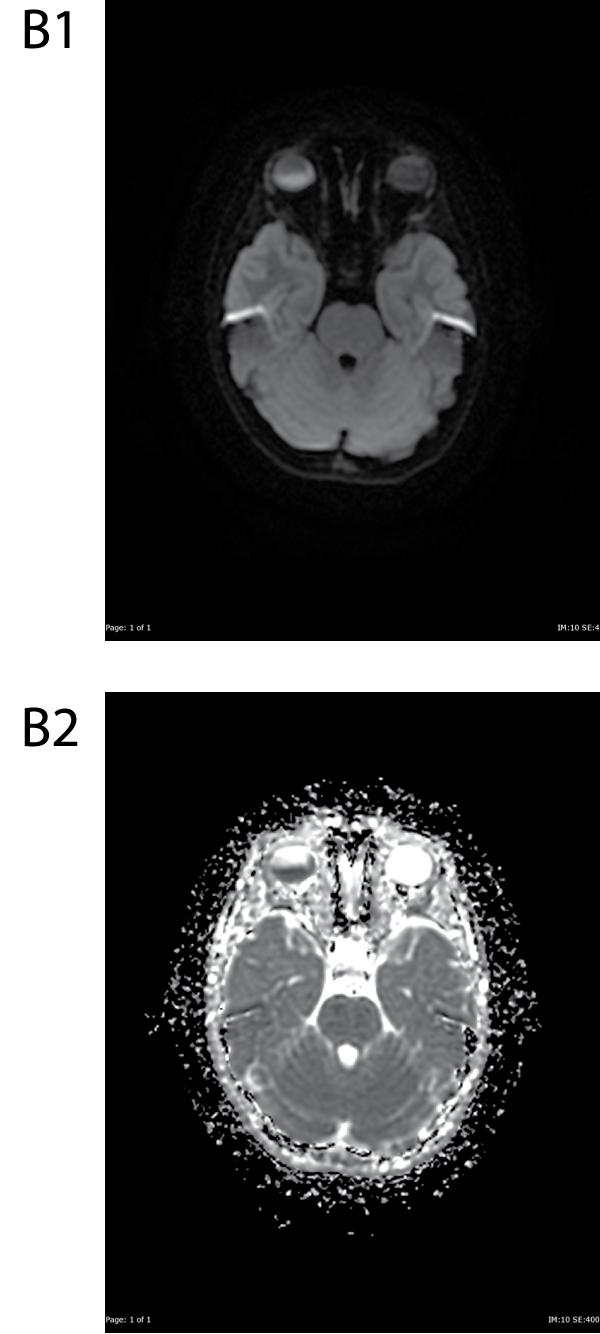
Figure B1&B2: DWI and ADC images show restricted diffusion within the posterior component of the mass.
View Figure 3
Lumbar puncture and bone marrow aspirate were performed, and revealed normal cerebrospinal fluid and 5% leukemic lymphoblasts in the bone marrow aspirate. Needle biopsy from the right eye was also obtained and sent for flow cytometry, revealing presence of pre B lymphoblasts. A diagnosis of CNS relapse of ALL was made and she was treated with multiagent chemotherapy and subsequently, radiation therapy and is currently in complete remission. When last examined, her vision in the right had improved to 20/25 without afferent pupillary defect. Left remained stable at 20/20.
Discussion
Orbital lesions comprise a heterogenous group of disorders: inflammatory, infectious, benign neoplasm, malignant primary neoplasm and metastases. Conventional imaging has been successful in identifying these lesions; however, the differentiation of malignant and benign lesions remains a challenge. With routine imaging modalities, findings often overlap and are nonspecific [1,5,6].
DWI has emerged as a useful tool in differentiating malignant and benign lesions using the measurement of apparent diffusion coefficient (ADC). This technique has been used in the identification of densely cellular tumors such as lymphoma, as well as other masses in the head-and-neck, such as cervical lymph nodes, parotid gland and thyroid nodules. There have been studies in the literature in recent years showing its efficacy in the characterization of extra-ocular orbital lesions [2,4-6].
Malignant lesions show a restricted pattern on DWI, leading to a lower ADC compared to benign lesions. This is secondary to the increased cellularity and enlarged nuclei seen in malignant lesions that lead to a decrease in extracellular matrix with subsequent reduction in the diffusion space of water protons in the extracellular and intracellular space. The ADC has also been used to further divide malignant lesions in terms of differentiation of tumor, with poorly differentiated tumors having more restricted diffusion secondary to higher cellularity. The ADC we measured in this lesion is similar to those reported in the literature for CNS lymphoma [7].
In our case of a rare, intraocular relapse of ALL, a combination of diffusion weighted imaging with gadolinium enhancement properties in the right globe helped lead to prompt diagnosis and treatment. The presence of restricted diffusion in the posterior right globe suggested a densely cellular process, such as lymphoma. Bland hematoma still needed to be excluded from the differential diagnosis, as hematoma can also cause DWI hyperintense signal [8]. Furthermore, this particular patient had subretinal hemorrhage seen on her eye exam and also had a personal history of spontaneous retinal hemorrhage in the other eye four years prior. Comparison of T1 pre- and post-gadolinium images showed uniform contrast enhancement of the posterior portion of the lesion, which excluded bland hematoma. This combination of findings on multiple MRI sequences strongly suggested intraocular malignancy and led to prompt initiation of invasive testing to confirm the diagnosis.
Acknowledgement
The authors would like to thank Dr. Reginald Sanders and Dr. William Deegan of Retina Group of Washington for their contribution in the care of this patient.
References
-
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45: 169-184.
-
Taylor CW, Taylor RE, Kinsey SE (2005) Leukemic infiltration of the orbit: report of three cases and literature review. Pediatr Hematol Oncol 22: 415-422.
-
Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, et al. (2010) Indeterminate orbital masses: restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology 256: 554-564.
-
Razek AA, Elkhamary S, Mousa A (2011) Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology 53: 517-522.
-
Kapur R, Sepahdari AR, Mafee MF, Putterman AM, Aakalu V, et al. (2009) MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol 30: 64-70.
-
Pui CH, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371: 1030-1043.
-
Stadnik TW, Chaskis C, Michotte A, Shabana WM, van Rompaey K, et al. (2001) Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 22: 969-976.
-
Silvera S, Oppenheim C, Touzé E, Ducreux D, Page P, et al. (2005) Spontaneous intracerebral hematoma on diffusion-weighted images: influence of T2-shine-through and T2-blackout effects. AJNR Am J Neuroradiol 26: 236-241.