International Journal of Blood Research and Disorders
The Study of the Mechanism of Action of the Newly Synthesized Direct Anticoagulant of Thiazoline Ammonium -4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate
Starkova AV,Syropyatov B Ya*, SobinFVandPulina NA
Department of Physiology, Perm` State Pharmaceutical Academy, Ministry of Health of the Russian Federation
*Corresponding author:
Syropyatov B Ya, Department of Physiology, Perm' State Pharmaceutical Academy, Ministry of Health of the Russian Federation, Russia, Tel: 8 (342)282-58-34; E-mail:allaperm@list.ru
Int J Blood Res Disord, IJBRD-2-006, (Volume 2, Issue 1), Case Report; ISSN: 2469-5696
Received: January 16, 2015 | Accepted: February 06, 2015 | Published: February 08, 2015
Citation: Starkova AV, Syropyatov BY, Sobin FV, Pulina NA (2015) The Study of the Mechanism of Action of the Newly Synthesized Direct Anticoagulant of Thiazoline Ammonium -4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate. Int J Blood Res Disord 2:006. 10.23937/2469-5696/1410006
Copyright: © 2015 Starkova AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of this research is to study the mechanism of action of the newly synthesized substance - Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate (FS-169), which possesses the direct anticoagulant activity. The research was conducted with the use of «?PG4-02-P» coagulometer. For the purposes of the study the rabbit plasma was used. The results show that FS-169 effectively changes Partial thromboplastin time and prothrombin time indicators. However, FS-169 has no influence on Thrombin time. The substance also does not affect the activity of II and V coagulation factors. FS-169 effectively changes the activity of the X coagulation factor.
Keywords
Direct anticoagulants, Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate
Introduction
Thromboembolic sequelae are a frequent cause of illness and death everywhere around the world. Venous tromboembolism is occurs in 100 – 160 people out of 100 000 of total annually [1].
Now a days the most perspective medicine for thrombosis prevention is the direct anticoagulants - direct inhibitors II factor (dabigatranetexilate) [2] and X factor (apixaban, rivaroxaban) [3,4]. However all these anticoagulants have certain disadvantages?
For example, bleedings of any localization, anemia, thrombocytopenia and liver dysfunctions are possible when using dabigatranetexilate. Contraindications to its use are severe renal insufficiency and liver dysfunctions [1,5].
When rivaroxaban was applied the following side effects, mentioned in the instruction for use, were identified – bleedings of any localization, anemia, thrombocytosis, tachycardia, hypotension, nausea, stool dysfunctions, stomachache, dry mouth, vomiting, increase in the activity of some enzymes, liver dysfunctions, dizziness, headache, faintings, renal insufficiency, itch, rash, extremity pain, fever, swellings andoverall deterioration of health. Contradictions to its use are bleedings, liver diseases, pregnancy, lactation period, child and teen age, severe renal insufficiency, lactose and galactose intolerance [4,6].
In the instruction for use to apixaban the following contradictions are stated: bleedings, liver diseases, renal dysfunctions, age below 18, pregnancy, lactation. The use of apixaban can cause anemia, thrombocytopenia, arterial hypotension, and bleedings of any localizations, nausea and liver dysfunctions [5,6].
Hence, the search for new compounds with direct anticoagulant activity is an urgent problem.
As the result of research into the compounds synthesized in Perm Satate Pharmaceutical Academy the new substance - Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate, which significantly lengthens blood clotting time for rabbits and dogs in vitro, was discovered [7]. It acts in vivo upon intragastric and subcutaneous administration to rabbits [8].
Therefore, the FS-169 can be administered orally and subcutaneously.
The aim of our research is to investigate the mechanism of action of the synthesized for the first time substance of Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate (FS-169).
Materials and Methods
The research was conducted on purebred rabbits 2-4 kg of weight. The overall quantity of rabbits was 85. Protocol number of the ethical committee which approved the research plan is ?67 from 4 November 2012. For the purposes of the research we used a «?PG4-02-P» coagulometer, a set of reagents for the Partial thromboplastin time (Ptt) and prothrombin time identification of AMKO’s production and sets of RENAM’s reagents for the Thrombin time and II, V, X factors identification. The blood was from a vein on a rabbit’s ear into the test-tube containing 3,8% citrate solute (9:1 v/v). The blood was centrifuged with 3000 rpm for 10 minutes. The plasma short of platelets was thus derived.
For the Ptt investigation 50 µl of rabbit’s plasma, 50 µl of 0,2% FS-169 solute were brought into the cuvette coagulometer, for the purposes of control instead of the substance 50 µl of isotonic NaCl and 50 µl of the reagent were added. The samples were incubated in +37ºC for 180 sec. after that 50 µl of 1% CaCl2 warmed up to +37ºC were added to the cuvette and the clotting time was then mesured.
For the prothrombin time measuring 50µl of the plasma and 50 µl 0.2% FS-169 solute were added to the cuvette coagulometer, for the purposes of control instead of the substance 50µl isotonic NaCl were added. The samples were incubated in +37ºC for 60 sec., afterwards 100 µl of warmed up in +37º C reagent was added and them the measuring took place. The apparatus automatically estimated prothrombin time (PT), prothrombin index (PI), prothrombin ratio (PR) and International Normalized Ratio (INR).
For the Thrombin time investigation 50µl of the plasma and 50µl of 0.2% FS-169 solute were added in the cuvette coagulometer, for the purposes of control instead of the substance 50µl of isotonic NaCl were used. The samples were incubated in +37ºC for 120 sec., after that 50µl of the regent was added and clotting time was estimated.
For the II, V, X coagulation factors activity identification 50 µl of the investigated rabbit plasma, 50µl of plasma deficient in factors and 50µl of 0.2% FS-169 solute were put in the cuvette coagulometer, for the purposes of control instead of the substance50µl of isotonic NaCl were used. The samples were incubated for 120 sec. in +37ºC, 100µl of the reagent were added and then clotting time was measured. The percentage of X coagulation factor was estimated automatically with the apparatus. The obtained results were processed by the Student-Fisher method [5].
Results and their Discussions
During the study of the FS-169 influence on Ptt, prothrombin time and Thrombin time indicators the following results, presented in Table 1, were obtained.
![]()
Table 1: FS-169 influence on Ptt, prothrombin time and thrombin time.
View Table 1
As is shown by the results, FS-169 substance effectively changes the prothrombin time and Ptt indicators. On the Thrombin time FS-169 has no influence. Therefore, FS-169 solute influences internal and external mechanisms of the I stage of coagulation –thromboplastin formation. The common factors of these mechanisms are II, V, and X. Thus we were able to identify the influence of FS-169 on the activity of these coagulation factors.
The obtained results are represented in diagram (Figure 1) and in Table 2.
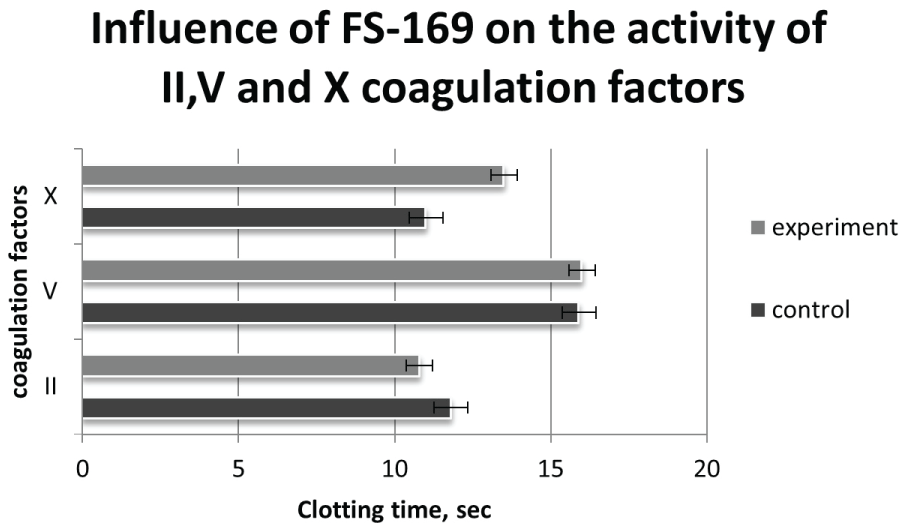
Figure 1: p- in comparison with control
View Figure 1
![]()
Table 2: Influence of FS-169 on the activity of II, V and X coagulation factors.
View Table 2
As is seen from the results, the substance does not influence the activity of II and V coagulation factors. FS-169 effectively is lowered the activity of X coagulation factor.
Conclusion
1. The Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate compound effectively lengthens Partial thromboplastin time and prothrombin time.
2. The Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate compound does not influence Thrombin time.
3. The Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate does not influence the activity of II andV coagulation factors.
4. The Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate compound effectively changes the activity of X coagulation factor.
References
-
Blommel ML, Blommel AL (2011) Dabigatran etexilate: A novel oral direct thrombin inhibitor. Am J Health Syst Pharm 68: 1506-1519.
-
Mak KH (2012) Coronary and mortality risk of novel oral antithrombotic agents: a meta-analysis of large randomised trials. BMJ Open 2.
-
Nutescu E (2012) Apixaban: a novel oral inhibitor of factor Xa. Am J Health Syst Pharm 69: 1113-1126.
-
Paikin JS, Manolakos JJ, Eikelboom JW (2012) Rivaroxaban for stroke prevention in atrial fibrillation: a critical review of the ROCKET AF trial. Expert Rev Cardiovasc Ther 10: 965-972.
-
Prozorovsky VB (2007) Statistical processing of the results of pharmacological studies /V.B. Prozorovsky// Psychopharmacology and biological narcology 7: 2090-2120.
-
Peradayn NA. New peroral antycoagulants/ N.A. Peradayn, N.V. Shuvalova
-
The Health of The Chuvash Republic 2013-14.
-
Starkova AV, Sobin FV, Syropytov BYa, Pulina NA (2013) Comparative study of the effects of Thiazoline Ammonium 4-Chlorophenyl-2-Hydroxy-4-Oxo-2-Butenoate and heparin on blood coagulation. Experimental and clinical pharmacology 76: 25-26.