International Journal of Brain Disorders and Treatment
Cranial Electrotherapy Stimulation (CES) in Neuropsychiatry: Definition & Insights from Electric Learning Paradigms
Naisberg Yakov*
AMCHA- Netanya branch, National Israeli Center for Psychosocial Support of Survivors of the Holocaust and the Second Generation, Israel
*Corresponding author:
Naisberg Yakov, AMCHA- Netanya branch, National Israeli Center for Psychosocial Support of Survivors of the Holocaust and the Second Generation, Israel, E-mail: naisberg@012.net.il
Int J Brain Disord Treat, IJBDT-1-004, (Volume 1, Issue 1), Research Article; ISSN: 2469-5866
Received: July 30, 2015 | Accepted: September 08, 2015 | Published: September 10, 2015
Citation: Yakov N (2015) Cranial Electrotherapy Stimulation (CES) in Neuropsychiatry: Definition & Insights from Electric Learning Paradigms. Int J Brain Disord Treat 1:004. 10.23937/2469-5866/1510004
Copyright: © 2015 Yakov N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The main point here is that CES method employ at both practices systematic applications of non-invasive, harmless electric currents in the range of 50 micro-to 2 milliamper, varying from 10 Hz to 100 kHz frequencies. They inject from 10 ms to 250 ms by each scalp pair out of an array of EEG electrodes transporting to regions of interest. Such electromagnetic forces induce equivalent number of ionic oscillatory streams crossing the brain in calibrated coulombs. Optimal control regulates by number of personalize treatment sessions reaching transitory homeostatic resynchronizing (THR) effects in electrical stimulated points limited to any electrode pair respectively. During THR neurotransmitters turnover normalizes liquidating neurological and psychiatric symptoms.
Objective: Review CES scientific articles over a period of 20 years to gain the insight of its electric mechanisms for improvement.
Method: Clinical CES procedures have been reviewed, analyzed and described.
Results:
Key hallmarks which emerge in CES efficacy cover :
I. Electric therapeutic agents (ETA) expressed in quantifying coulomb charges.
II. ETA modes for resynchronizing and desynchronizing neuronal membraine ionic Equilibrium, lipid dielectric properties and neurotransmitter's turnover.
Conclusion: CES is a safe, non-invasive, reliable and measurable procedure having dose-related units for treating mental and physical disorders of interest.
Keywords
CES, Ionic Equilibrium, Neuronal Electrical Resynchronization.
Introduction
The scope of this article is design tp elucide CES quantitative treatment's dosimetric levels regarding diagnostic and treatment efficacy managing psychiatric and neurological diseases [1,2]. Actually, in both disorders the breakdown in neuronal electric equilibrium is the most likely factor participating in brain's quuntify biochemical and biophysical disturbances [3,4]. A way to partly overcome this problem lays in the administration of specific CES quantitative dosimetric patterns enabling primarily changing biophysical and biochemical relationships in affected neurons causing prominent effects in the course of treatment. Today, its specific dosimetric mechanism and effect of action is still unknown to the scientific community [5].
The cardinal point here is that effects of psychotropic medicine and electrotherapy show an identical improving effect on certain mental disorders [6]. Furthermore, it seems likely that electrical dosimetric forces like drug dosages are responsible for changes in the brain that eventually favorable influence Mind operations [7]. CES electric considerations are limit to dosimetric expressions producing trends for neuronal functional changes. To fulfill a competent role the electric forces must have unique biophysical characteristics for producing the great therapeutic benefit.In this respect, George et al. [8] pointed out that electric and magnetic stimulation influence the intrinsic neuronal electrical properties. The logic of this conclusion assumes that any shift in the physical configuration, density and shape of ion channels on neuronal membranes may change their electrical properties providing alter responses to outer electrical stimulation [9]. As these manifest, a growing need emerges for a base-evident theory unfolding the therapeutic biophysical mechanisms of the neuronal electric behavior to elicit its quantiquamntify dosimetric units [10,11].
A complex neuronal network influenced by external electric forces allow responses for variability due to altered intrinsic neuronal electrical properties. However, against this background a conceptual model will suggest to connect the neuronal networks electrical control over basic homeostatic synchronizing factors controlling a) all organs and systems of the unifying organism and b) the inner-outer information exchange. A critical point underlining it is that these neuronal networks represent an intricate bioelectrical controlling system [12,13] composing of :
• discrete wire-analog autonomous and mental communication netting;
• inherited electrical forces governing the netting homeostatic sustenance;
• electrical properties regulating the netting biophysical information-processing.
The solution to the question of what model to employ in order to clarify the nature of CES and its benefits must be explained from electric resynchronizing benefits playing a central role in correcting neurotransmitters turnover. This likely neuronal electrical mechanism must have universal features that can be restored under the influence of outer quantitative dosimetric electric currents [14]. With the impact of this mechanism, one may state that a significant context in which to view CES dosimetric electric currents is the number of CES sessions needed for remodeling the neuronal wiring netting replacing electric desynchronizing with resynchronizing rhythms to facilitate the previously hindered information processing. Thus, the aim of this article is three fold to :
• Show that the electrodes metallic electrons flows carry on electrical dosimetric forces to scalp regions, enabling mediating equivalent cerebral ionic charge flows producing electrical therapeutic agents (ETA) influencing cross sectional neuronal tissues in places of interest.
• Define ETA data from CES procedures providing a therapeutic efficacy.
• Present the biophysical (electrical) mechanisms of ETA enabling from afresh resynchronizing affected excitable neurons with cooperative electrical stimulating effects permitting an optimal CES procedure emergence.
Electrical Therapeutic Agent (ETA) : Definition and Mechanisms of Operation
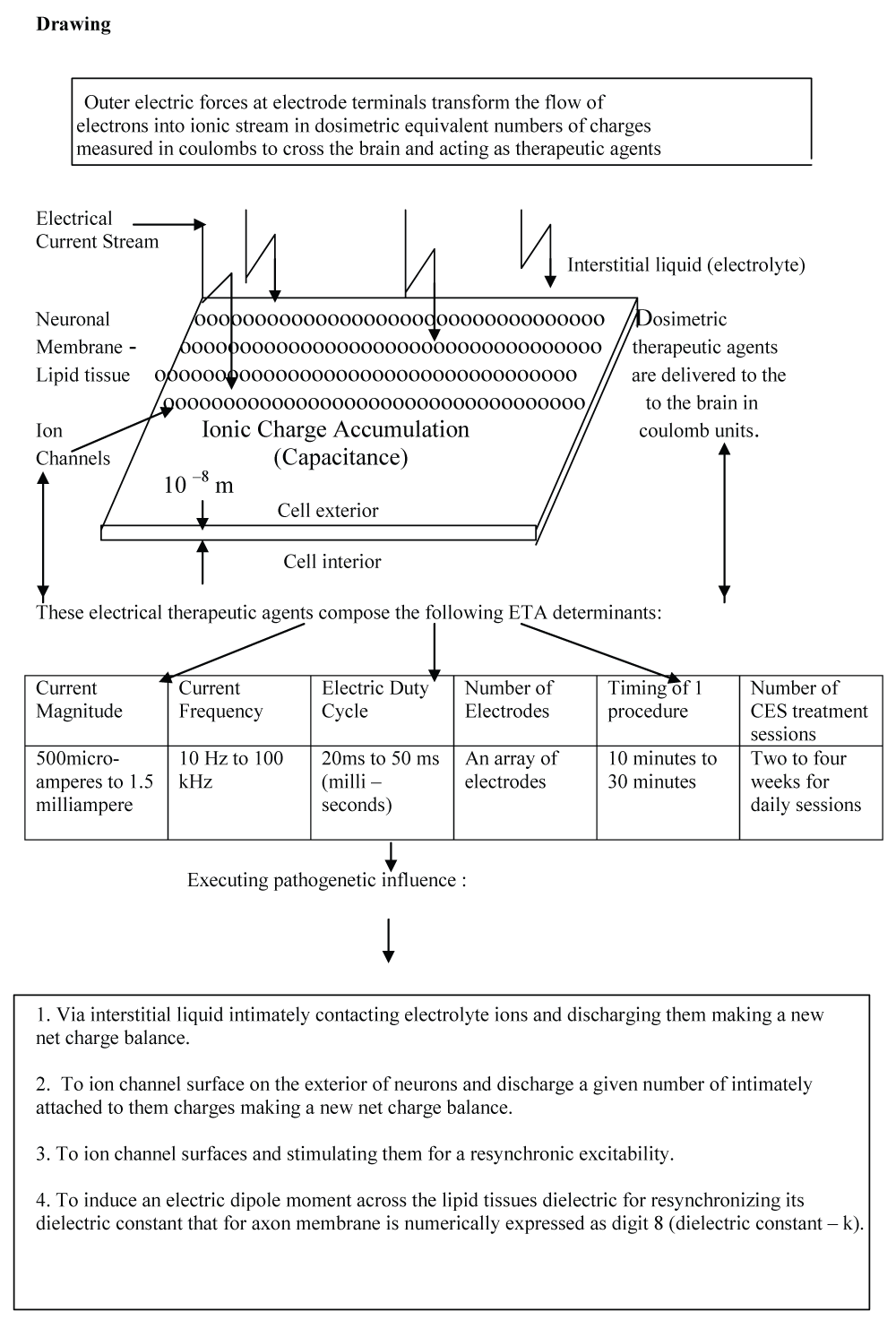
ETA defines an induced electromagnetic directed stream of ionic charges measured in coulombs deriving from and linked with inner electromagnetic induction generated from outer electric currents applied to pairs of electrode terminals. The latter have been placed over wet or covered with gel scalp regions to resynchronize the electrical desynchronizing properties of cross sectional brain tissues of interest. ETA is responsible for the fundamental interactions controlling electrical forces in atoms, chemical reactions and electromagnetic phenomena [15].
The principal point her is that to confirm ETA phenomena, one must have a clear knowledge about the nature of electrical and magnetic forces and fields. It is proven by experiments that two or more electrical charges exert forces upon each other. Two identical charges repel and two opposite charges attract each other [16]. In the same way two and more electrical charges produce electrical fields in their proximity. So any charge in accord with the third Newton's law will experience a force (F) to be proportional to the electric field (E) and to the charge itself, F = q E. In which the positive charges experience forces parallel to the field and negative charges to the contrary. From the electrical field perspective E = NC-1. Accordingly, N is the force in newtons and C is the magnitude of charges in coulombs. Electrical charges on electrons travel in metallic conductors with a speed of light, c = 3 x 108 ms-1, whereas a neuronal ionic pulse is one millionth that of a pulse in metallic wires. Traveling charges produce electrical currents and the latter produce in its vicinity magnetic fields. The latter can induce proportional electric current parameters beyond the skin electrolytes when electrodes are tightly contacting the wetted skin area.
Electrical forces sustain the motion of electrons in their orbits in atoms in a similar way as gravitational forces are accountable for keeping planets in their orbits. Gravitational forces have related particles expressed in mass and electrical forces contain electrical charge units, as well. Electric charge fundamentals are [16,17]:
• A resting mass of an electron having 9, 1093897 (54) x 10-31 kg representing a basic particle of the matter which integrally rotates around the nucleus of atoms.
• Having small charged fractions on electrons to be been named quark.
• When detached from atoms becoming free electrons constituting the electrical current stream.
• In case of an excess of electrons on a given body it may become negatively charged, as contrasted with an excess of protons providing positive charges.
• Defining the standardized unit of charge is a coulomb and knowing that 1.602 × 10-19 coulombs of charges are on a single electron.
• Having on Na + ion an equal to electrons charges.
• Having electric potential which relates to the potential energy for per unit of charge and can be linked to the position of interest on neuronal membranes.
(i) Having a potential energy equation of :
(ii) Having an operable equation :
• Having resting charges to produce an electric field that points away from the positive and points to the negative charges, as contrasted with charges in motion that produce magnetic fields.
• Having charges tot oscillate forth and back to create electromagnetic waves in alternating currents that carry on the programming of information in coded forms.
• Having charge carriers, electrons in metal conductors and ions in electrolytes.
• Having electric repelling forces for two identical and attractive forces for two opposite charges.
• Having a remaining constant net charge when charges are destroyed (discharged). It means that when negative charges are destroyed with electrical current streams an equal number of positive charges become spontaneously destroyed as well.
This phenomenon leaves the net charge unchanged and its is called the conservation of charges and ions are accountable for the overall neuronal electrical phenomena. Having charge density for per unit of volume of a medium or per unit of surface area of a body. The higher the charge density the more is the electrical strength. n an outer electric circuit limited to two EEG electrode terminals, the electrical forces and current when applied to the wet (or with gel) scalp region of interest produces instant alternating magnetic fields that, in turn induce the creation of ionic currents from scalp and brain tissue directing it to the opposite electrode. These ionic charges obtain equivalent dosimetric electric forces that exists on terminals of any pair of electrodes to compose the fundamental ETA expressions. Once outer electricity is applied to biological tissues including the brain it never conducts metallic electrons inside living tissues, but mediates the physical (electrical) forces driving activated ions across tissues to be located between a pair of electrodes.
Thus, outer induced electromagnetic forces enable to mobilize a local stream of ions having ETA faculties from the act of flow from negative to positive charged terminals of electrodes in direct currents and shifted positions in alternated currents respectively. It is know from Physics [16] that two elementary particles in nature, electrons and protons of atoms bear an equal amount of negative and positive charges. That's why it is necessary to study neuronal ionic behavior with learning paradigms obtained from electrons flows in metallic conductors. The s principal point here relies on knowledge of how charge particles residing on ions exert the same quantifying dosimetric effects that have been induced by metallic electrons delivered to the electrode terminals applied to the scalp. Our assumption is that electric charges can be quantified in coulombs acting like drug dosimetric units and can be precisely measured with temporal gradation [17,18].
Electric Therapeutic Agents (ETA) Composites
There is much fascinating drawing to introduce, if one can only overcome the explanatory obstacles presenting the ETA phenomenon. By using a technological model one can demonstrate ETA parameters in the following drawing.
A subject undergoing CES treatment with 1, 5 mA external current and 10 Hz frequency applied to the brain with 10 ms (milliseconds) duty cycle and 10 ms out of duty through a pair of electrodes during a therapeutic session of 10 minutes will obtain from the formulae Q = I x t ( Q is the amount of charge, I is the magnitude of current and t - is the timing of the procedure) - 4.5 C (coulombs). The calculation is as follows : for a 10 minute CES procedure the duty cycle is 5 min.,then 1.5 mA 5 x 5 min = 1.5 x ( 5 x 60) = 1.5 x 300 sec = 4500 : 1000 = 4.5 C. If the same CES procedure lasts about 30 minutes then 4.5 x 3 = 13.5 C. of charges needed to receive a given therapeutic effect in each CES session.
A Conceptual Model for CES Usage
The critical point here is that we proceed to introduce the basic CES [19] elements to gain a reasonable level of understanding of the role of electrical parameters able to exert a favorable effect. The entity that executes a therapeutic effect is the 'stream of ionic charges' expressed in coulomb units for therapeutic efficacy. This 'stream of ions ionic charges' provide the (electrical) modulation units on brain electric tissues. Thus, the greatest impact of any applied electrical current is its ability to produce safe electrical forces acting upon scalp regions to induce ionic stream flows from scalp through brain tissues to create therapeutic ionic circuits. The number of Coulombs that interact with impaired electrical properties of given neurons will define the unit of a therapeutic effect in the region of interest.
CES uses low and high frequency of electric currents applied to the frontal and temporal regions of the scalp. It was noticed that CES has following merits [20]:
• Non invasive.
• Efficient.
• Possess health benefit.
• Convenient.
• Safe in usage and contain no health threat.
• Applying mild battery-powered electronic stimulation.
• Simple in operation.
• Comfortable.
• Compact.
• Portable.
The conceptual basis for CES utility is to reclaim control over [21,22]:
• Anxiety.
• Depression.
• Schizophrenia.
• Attention deficit hyperactive disorders.
• Pain.
• Headache.
• Stress and stress-dependent disorders.
• Substance abuse- drugs, alcohol, and smocking.
• Insomnia.
The key point here is that CES achieves brain's biochemicak resynchronizing effects [23,24]:
• Increases conversion of amino acids into
• molecules forming neurotransmitters.
• Rebalance neurotransmitters release.
• Reclaims alpha and delta brain waves.
• Raises blood levels of endorphins.
• Reduces spasms and edema.
• Tonifies weak muscles.
• Achieves deep relaxation.
It is also reported that CES improves [25,26]:
• Memory.
• Concentration.
• Mental clarity.
• Learning.
• Vitality.
CES sets up a complex cooperative electric mechanism influencing the impaired electrical faculties of neurons of interest. For these reasons dosimetric quantities are of critical importance in achieving a desirable therapeutic effect. The central point here is that CES must fulfill the following prerequisites:
• It must have outer electric current magnitudes defined in micro or milliampers units.
• It must rely on high frequencies having no subjective side effects.
• It must be of optimal timing exposition to injected charge units.
• It must move through an electric conductive medium to accomplish an electric artificial circuit build-up.
• It must have the needed electrical parameters accurately influencing electrical desynchronized properties of neuronal electric tissues.
• It must display the number of singular CES procedures needed to bring one's
• relapse into a remission state of a given disease.
• It must hold personalize predictive parameters for healthy states.
• It must display the optimal electrical parameters useful for preventive needs.
CES methods of research require real CES and Sham CES application for monitoring the utility and efficacy of CES intervention with manipulating dosages for disclosing the optimal electrical parameters showing an optimal individual response.
Prospectives of CES Resynchronizing Impaired Neuronal Electrical Properties
The major articulating point here is that it focuses on the fundamental understanding of how CES procedures efficiently influences Autonomous Neuronal Networks (ANN) and Mental Neuronal Networks (MNN), respectively [27]. Given this demonstrated efficacy and safety, one should assume that neuronal membranes electric impaired faculties could be restored with the assistance of outer electrical forces. It frequently points out that these electrical homeostatic properties accurately influence ANN and MNN electrical wiring systems. We may present the neuronal netting architecture and function having [28]:
• Wire-analog ion channels with electrical excitable qualities.
• Self-arranged ion channels to create linear neuronal electric pathways.
• Ion charge exchange through passive ion channels of diffusion to sustain an ongoing ionic equilibrium.
• Action potential propagation via ionic pumps.
• Normal electrical threshold level.
• Normal excitability of quantifying neurotransmitter vesicles.
• Normal dielectric property of lipid membranes.
For learning paradigms it is proper to consider that these netting-like technological elements could be worn out and broken. Here one might consider the altered neuronal electric properties to be parts of netting impairment. This yields a corresponding defect in the neuronal electrical generation system [29]. Above all, emphasis here are on the emergent disequilibrium in ionic charges sustaining electrical impaired functions across certain brain regions. Electrical impairment is an expression of disconnection and miscorrelation in certain neuronal zones of electric activity [30]. Correspondingly, the principal aim of CES is to restore the ionic equilibrium across ANN and MNN leading to a fresh emergent transitory homeostatic resynchronization (THR) remission. Thus, CES represents a fresh model of usage of electrotherapy that embraces biophysical mechanisms underlying electric resynchronizing modulation with four basic components:
• The magnitude (amplitude, density) of applied charges.
• The frequency of charge waves delivery.
• The cycling time of current application.
• The number of EEG electrode pairs applicable to regions of interest.
Thats why it is important to bear in Mind that CES therapy provides an instant dosimetric medium resembling drug dosages mediating electrical charges through non-invasive cerebral tissues to solve complex neuronal electric problems. Such problems is not easy to solve, since CES currents are volume distributed.Other features include the conductivity direction and electric current transportation between any pair of acting electrodes. Hence, there is no linear relation between the current that is emitted from electrode and delivered to another electrode. It must be stated that a general brain conductivity medium exists between any pair of acting electrodes in which electrical currents run through cerebral liquid and bulk tissue, accordingly. Even in an intact brain the electric current mainly selects pathways having the least resistance to its conductance. One way of understanding the influence of these neuronal electric problems is to accept as an axiom that the brain in itself is a complex electric organ containing inner self-capacitance enabling electric generation that comes in an interaction with any applied electrical current. Once we know that brain tissues can undergo changes, we may grasp the notion that neuronal electrogeneting shifting may appear as well. In that case one would expect that applied currents may profitable influence and control the altered neuronal electrogenerating properties [31].
Strikingly, novel ideas relate to the nature of electrical interaction of applied currents with neuronal tissues, embracing the emerged electrical behavior of neuronal tissues that have been under different specifications. Traditionally, it is known that the passage of applied electrical currents through healthy or morbid neuronal tissue have contrasted electrical calibration. Hence, learning principles highlight some current ideas of how outer electric current calibration can help in identifying levels of neuronal elecrogenerating alterations. The most important point here is that CES, in general mediates different electrical parameters that, in turn induce a variety of neuronal electric behavior to be displayed on local spatial distributions. As previously mentioned, the goal of this article is to show that CES introduces an electricals resynchronizing mechanism the biophysics of which postulate that ion channel matrixes primarily reshape neuronal (ionic) electrical waves to recapture from a fresh the natural synchronic pace causing proper brain electrical conductance. From this point of view, if one can look at all interrelated biochemical and biophysical aspects of neurons, it must be clear that the electrical attuning factors of the brain neurons must be actively driven into a fresh homeostatic resynchronization. It means that the general rules of Physics operating in the human brain assist in resynchronizing previously desynchronized electric properties. Concurrently, CES tunes into these biophysical principles to augment electric effects on the neuronal electric architecture and its neuronal own electrical function. Thus, sooner or latter, after a serial of CES applications the brain tissue may functionally enter its electrical resynchronizing phase due to environmental demands. Certainly, it is thus instructive to state that the realization of CES initiates an electrical refreshing operating regime across given ANN and MNN driving them into an anticipated THR remoission. The assumption underlying this statement is that the electromedicine used in CES procedures culminates in resynchronizing primarily the electrical faculties of neurons driving them further to restore their ionic equilibrium with an adequate pace for a new transitory homeostatic resynchronization arrival.
Conclusion
While there remain a number of challenges ahead, it is obvious that one must understand the basics of neuronal electric desynchronization prior to the usage of electric therapeutic agents (ETA). Four cardinal electrical mechanisms participatin in ETA formation are [32,33]:
• density of ionic charges generated by the applied currents.
• frequency of ion charges for given units of time.
• duration of ionic charge introduction.
• cross sectional distribution via neuronal conductive routes.
ETA formation is presented by quantifying units in coulomb charges, easy calculated from the formulae Q = I x t. Any CES procedure can be quantified in coulombs. CES equivalently induce withy metallic electrons an equivalent intensity and frequency of biophysical ionic streams undet the same number of Coulomb charges operating from the soft scalp tissue and dura matter through cerebral liquid and cerebral tissue acting upon:
• Instant discharge of local counter charges that have been attached to both surfaces of neuronal membranes converting them into neutral particles.
• Local desynchronized ion channel tissues driving them to recapture normal electric (ionic) excitable properties.
• Local membrane lipids causing lipid composition changes that further indirectly brings dielectric property changes.
• Indirectly re-establishing ionic equilibrium on given neuronal conductive pathways leading to an electric transitory homeostatic resynchronization (THR).
The key issues of CES is the challenge to define the number of coulombs exerting clinical efficacy of a single session. It is true by definition that duration of CES introduction differs from state-to-state. The actual mode of stimulation can be condensed or extended in units of time. If this is so, one can anticipate short-term and long-term CES sessions leading to different outcomes. As for an optimal model development further research must challenge the number of coulomb charges used in single sessions, the extension of a session, and the number of sessions for each given subject and illness entity. With this in mind we believe that CES having an additional dosimetric diagnostic assessment may broadly enter neuropsychiatric practice.
References
-
Kirsh DL (2002) Science Behind Electrotherapy Stimulation. Medical Scope Publishing Cooperation, Edmonton, Canada.
-
Li S, Zaninotto AL, Neville LS, Wellington SP, Nunn D, and Fregni F (2015) Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence. Neuropsychiatr Dis Treat 11: 1573-1586.
-
Rossignol DA, Frey RE (2012) A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation inflammation, oxidate stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 17: 389-401.
-
Brennan BP, Rauch SL, Jensen JE, Pope Jr. HG (2013) A critical review of magnetic resonance spectroscopy studies of obsessive -compulsive disorder. Biol Psychiatry 73: 24-31.
-
Gershon AA, Dannon PN, Grunhaus L (2003) Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 160: 835-845.
-
Jutzeler CR, Curt A, Kramer JL (2015) Effectiveness of High-Frequency Electrical Stimulation Following Sensitization With Capsaicin. J Pain 16: 595-605.
-
Oken BS (2005) Complementary therapies in Neurology: An evidence - based approach. The Pathenton Publishing Group, New York
-
George MS, Lisanby SH, Sackeim HA (1999) Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 56: 300-311.
-
Li Y, van den Pol AN (2009) Enhanced excitatory input to melanin concentrating hormone neurons during developmental period of high food intake is mediated by GABA. J Neurosci 29: 15195-15204.
-
Hamdy S, Aziz Q, Rothwell JC, Hobson A, Barlow J, et al. (1997) Cranial nerve modulation of human cortical swallowing motor pathways. Am J Physiol 272: G802-808.
-
Gronlund J, Jalonen J, Valimaki I (1997) Transcephalic electrical impedance provides a means for quantifying pulsatile cererbral blood volume changes following head - up tilt. Early Hum Dev 47: 11-18.
-
Bressler S (2002) Understanding cognition through large-scale cortical networks. Curr Dir Psychol Sci 11: 58-61.
-
Campo P, Poch C, Parmentier FBR, Moratti S, Elsley JV, et al. (2010) Oscillatory activity in prefrontal and posterior regions during implicit letter-location binding. NeuroImage 49: 2807-2815.
-
Datta A, Bikson M, and Fregni F (2010) Trabscranial Direct Current Stimulation in Patients with Scull Defects and Skull Plates: High-Resolution Computational FEM Study of Factoes Altering Cortical Current Flow. Neuroimage 52: 1268-1278.
-
Bikson M, Datta A, Rahman A, Scaturro J (2010) Electrode montages for tDCS and weak transcranial electrical stimulation: role of "return" electrode's position and size. Clin Neurophysiol 121: 1976-1978.
-
Kane JW, Sterheim MM (1988) Physics. John Wiley & Son, New York.
-
Isaaks A, Daintith J, Martin E (1996) Oxford concise Scientific Dictionary. Oxford University Press, Oxford, New York, P: 297.
-
Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, et al. (2013) Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage 74: 266-275.
-
Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A (2011) Transcranial brain stimulation: clinical applications and future directions. Neurosurg Clin N Am 22: 233-251, ix.
-
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2: 145-156.
-
Couturier JL (2005) Efficacy of rapid-rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta-analysis. J Psychiatry Neurosci 30: 83-90.
-
Rossini PM, Rossi S (2007) Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 68: 484-488.
-
Feusner JD, Madsen S, Moody TD, Bohon C, Hembacher E, et al. (2012) Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav 2: 211-220.
-
Gilula M, Kirsch D (2005) Cranial electrical stimulation review: a safer alternative to psychopharmaceuticals in the treatment of depression. Journal of Neuro therapy 9: 7-26.
-
Crause B, Cohen Kadosh R (2013) Can transcranial stimulation improve learning difficulties in typical brain development? A future possibility for cognitive training. Dev Cog Neurosci 6: 176-194.
-
Lee IH, Seo EJ, Lim IS (2014) Effects of aquatic exercise and CES treatment on the changes of cognitive function, BDNF, IGF-1, and VEGF of persons with intellectual disabilities. J Exerc Nutrition Biochem 18: 19-24.
-
Pereira A Jr (2003) The Quantum Mind/Classical Brain Problem1. NeuroQuantology 1: 94-118.
-
Sporns O (2013) Structure and function of complex brain networks. Dialogues Clin Neurosci 15: 247-262.
-
Robinson DL, Hermans A, Seipel AT, Wightman RM (2008) Monitoring rapid chemical communication in the brain. Chem Rev 108: 2554-2584.
-
Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, et al. (2010) Functional connectivity and brain networks in schizophrenia. J Neurosci 30: 9477-9487.
-
Zaghi S, Acar M, Hultgren B, Boggio PS, and Felipe Fregni F (2010) Noninvasive Brain Stimulation with Low-Intensity Electrical Currents: Putative Mechanisms of Action for Direct and Alternating Current Stimulation. Neuroscientist 6: 285-307.
-
Naisberg Y, Modai I, Weizman A (2000) The wired network as a learning paradigm for normal and abnormal brain neuronal communication. Med Hypotheses 55: 133-136.
-
Naisberg Y (2000) Biophysical body-brain-mind unity: theory and practice. Med Hypotheses 54: 385-391.