T helper Regulation and Anti-Cytokine Therapy in Asthma
Zihan Zheng1* and Xinyi Huang2
1Department of Microbiology & Immunology, School of Medicine, University of North Carolina at Chapel Hill, USA
2Xiangya School of Medicine, Central South University, P. R. China
*Corresponding author:
Zihan Zheng, Department of Microbiology & Immunology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Tel: (646)-724-7378, E-mail: zihanz@live.unc.edu
Int J Aller Medcations,
IJAM-1-002e Volume 1, Issue 1,
Review Article,
Received: February 15, 2015: Accepted: April 13, 2015: Published: April 15, 2015
Citation: Zheng Z, Huang X (2015) T helper Regulation and Anti-Cytokine Therapy in
Asthma. Int J Aller Medcations 1:003
Copyright: © 2015 Zheng Z. This is an open-access article distributed under the terms of
the Creative Commons Attribution License, which permits unrestricted use, distribution,
and reproduction in any medium, provided the original author and source are credited.
Abstract
Asthma is a complex chronic inflammatory disease of the airways that is of increasing prevalence. Many different cell types are critically involved in its pathogenesis, including several classes of T helper cells. These cells may serve to generally organize the asthmatic response by virtue of the cytokines and other factors they release, which trigger downstream effects on a wide variety of cells. In this review, we cover some of the effects that these T helper cells may have, as well as the effect that different medications may have on these cells. In particular, we overview some of the anti-cytokine therapies so far conceived, and discuss some other possible avenues of approach that allow for selection against target cell types.
Keywords
Asthma, T helper, Anti-cytokine
Introduction
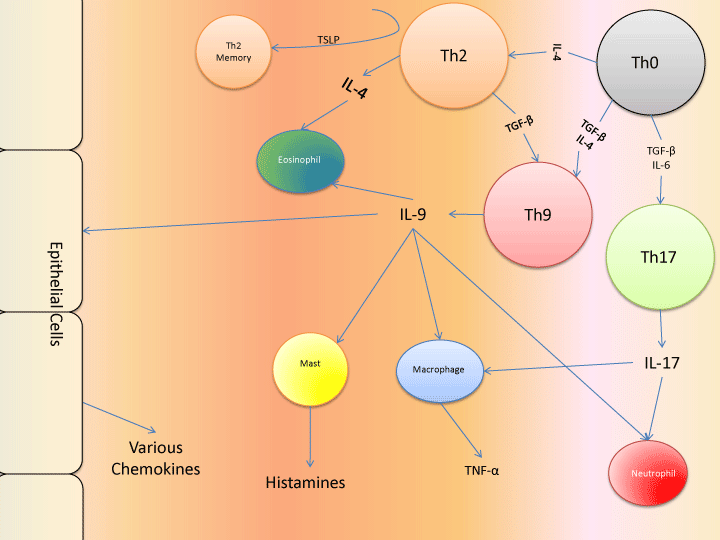
Asthma is a complex chronic inflammatory disease of the airways that affects over 1 in 12 individuals in the United States, and more than 230 million people around the world [1,2]. Characterized by airway hyperresponsiveness (AHR) and acute attacks of constricted breathing and swelling of the lungs, asthma can lead to permanent airway remodeling and may be life-threatening [3]. Several different conditions have been linked with asthma, with the most prominent of these being obesity [4]. The most common type of asthma is allergic asthma, in which the attacks are triggered by allergic reactions. While many medications have been developed to treat the condition, there remains no cure. Some of the commonly used management methods also have significant long term side effects, as they are mostly broad-functioning immunosuppressants (e.g. corticosteroids). However, deeper mechanistic insights into the condition may lead to the generation of more specific targets. In particular, T helper cells that organize and drive the immune response in asthma may be especially promising and meaningful targets for therapy. While AHR can sometimes be induced in the absence of T cells, it is clear that T cells drive significant effects when they do exist [5]. In this review, we focus on the roles played by several T helper subtypes in asthma, and the effects that various medications may have on these subtypes, with a particular emphasis on Th9, given the relative paucity of reviews on the subject relative to the importance of their impact. A pictorial summary of the interactions covered can be seen in Figure 1.
.
Figure 1: Network of major cell types and secreted products in asthma. It should be noted that specific monoclonal antibodies have been generated to act
against all of the secreted products, with most being unsuccessful. Anti-inflammatory cytokines and effects are omitted, as are autocrine loops such as those
involving IL-2, IL-5, and IL-7.
View Figure 1
Th2 Cells
Th2 cells were the first lineage of T helpers cells identified to be critical in asthma pathogenesis, as part of a paradigm in which Th1 cells mediated inflammation against foreign antigens while Th2 cells regulated autoimmunity. While the discovery of other T helper subsets have led to substantial revisions in the paradigm, the important role of Th2 cells in asthma has only been confirmed. Controlled by trans-acting T-cell-specific transcription factor GATA-3 (GATA-3), Th2 cells may differentiate in response to either interleukin (IL)-4 and IL-2, or IL-2, IL-25, and thymic stromal lymphopoietin (TSLP) [6]. The common denominator, IL-2, signals via activation of GATA-3 and signal transducer and activator of transcription (STAT)5, which have been shown to be vital for Th2 differentiation [7,8]. In fact, GATA-3 is understood to be the lineage-defining transcription factor for the phenotype [9,10]. GATA-3 undertakes that role by suppressing proteins that promote alternative fates, such as the Th1-linked STAT4 [11,12]. However, other proteins such as STAT6 and YY1 are also critical to successful Th2 differentiation [13].
Capable of producing cytokines such as IL-4, IL-5, IL-6, IL-9 and IL-25, Th2 cells also help control B cell immunoglobulin class-switching to the “allergic” IgE [14-16]. Th2 production of IL-4 is especially important for its role in inducing further Th2 and Th9 polarization. In addition, the assortment of cytokines that Th2 cells may release can influence the behavior of many other cell types, as reviewed elsewhere. Such class switching has been identified to be of significance in allergic responses [17]. Th2 cells also have an interesting plasticity dynamic in which they may take on an additional phenotype as a double positive population, a result first seen with Th2/Th17 cells, but which may also be extended to GATA3+ Tfh cells. Th2 cells have also been shown to differentiate into Th9 cells (or at least IL-9 producing cells) in the presence of TGF-β [18]. Studies have also shown that TSLP may be essential in stimulating long-term Th2 survival/memory, such that a significant pool of Th2 cells may form following an initial acute inflammatory event and then rapidly respond to a second inflammatory event by reaching the lungs, forming a vicious cycle [19]. While the precise duration of Th2 activity is still unclear, their memory capability is very relevant to understanding asthma pathogenesis.
Th9 Cells
Following the activity of Th2 cells, the Th9 subtype may then emerge at the site of inflammation to further modulate immune function. Th9 cells generally require the presence of IL-4 and TGF-β to polarize, with the two signaling pathways that follow from those cytokines dictating their polarization, although other cytokines such as IL-25 may also play supporting roles [20-23]. It is commonly understood that both IL-4 and TGF-β are present in significant quantities in asthma, and larger populations of Th9 cells have been reported in asthma patients, as well as in other related airway and autoimmune disorders (e.g. atopic dermatitis) [24-28]. Curiously, the reactive oxygen species NO may significantly up regulate Th9 cells contrary to its general inhibitory effects elsewhere [29]. However, Th9 presence in many cases may be somewhat brief, potentially due to the high impact it may have in the local environment [30]. While a skin-homing capability for Th9 cells has been reported, it is unclear precisely how they may also localize to the lungs, or how they may change to a memory state [31].
It is generally understood that IL-4 signals through the JAK/STAT, ERK, and Akt/PKB pathways, while TGF-β signaling involves SMAD family and Pu.1 [32-36]. These pathways interact with each other to suppress transcription factor associated with alternate fates, such as the Treg lineage-defining transcription factor Foxp3, and consequently drive Th9 lineage commitment. While no clear lineage-defining transcription factor has been identified for Th9 cells, such a protein would doubtlessly be related to those signaling pathways. What is clear is that the end result of the complex interactions that occur in Th9 cells is the recruitment of proteins to the IL-9 promoter, and subsequent transcription and secretion of the cytokine. The transcription factors IRF4 and Pu.1 have been identified to be especially critical for such transcription, and both can directly bind onto the IL-9 promoter [37-39]. As such, regulation of the two proteins may be of great utility in controlling Th9 activity.
First identified for their propensity to produce large amounts of IL-9, Th9 cells have since been shown to also produce significant amounts of several other cytokines of interest, namely IL-10 and IL-21 [40]. IL-9 is understood to be a largely pro-inflammatory cytokine, which may trigger signaling through the IL-9 receptor via JAK/STAT to drive NF-κb activation, among other direct and indirect effects [41]. IL-9 expression has been shown to be highly elevated in asthma, in both murine models and human samples. Mice deficient in IL-9 producing cells due to IRF4 knockout experience less severe asthma in the ovalbumin model [42]. Other reports have shown that IL-9 signaling is also important in the development of ulcerative colitis and other autoimmune disorders [43,44]. Many different cell types possess the IL-9 receptor, and several other cell types may also be modulated by IL-9 through other means. Of particular interest to asthma and allergy is the fact that the Th2 and Th17 subtypes express much higher amounts of IL-9R than other T helper subtypes [45]. In addition, neutrophils and mast cells also express the IL-9R, consistent with reports that those cell types can be strongly influenced by Th9 activity [46-49]. IL-9 has been shown to promote mast cell expansion and function, leading to the production of histamines that play a large role in the negative effects associated with asthma and allergies [50]. IL-9 may also enhance B cell IgG production in conjunction with IL-4 production by Th2 cells [51,52]. IL-9 may also play a role in the B cell selection process, given that its receptor is more highly expressed in germinal center (GC) B cells than circulating B cells [53]. Airway smooth muscle cells also express the IL-9R, and may have their chemokine release modulated by IL-9 [54]. Several reports have suggested that some macrophages and epithelial cells may also receive IL-9 signal transduction through means not currently understood [55,56]. IL-9 has also been shown to have an effect on cells less studied in relation to asthma [57,58].
IL-10, on the other hand, is an anti-inflammatory cytokine, whose production can lead to suppression of the TNF-a, IL-1b, and IL-6 pathways [59]. Such suppression runs directly contrary to the pro-inflammatory activity of IL-9, and might be used as a balance of sorts to prevent unregulated inflammation. IL-21 production by Th9 cells has been demonstrated to be important to the anti-tumor capabilities of Th9 cells, and may be thus less relevant to Th9 function in autoimmunity, although it should be noted that IL-21 can stimulate naïve T helper polarization into Tfh [60-62]. IL-21 can also influence B cell activity [63]. Beyond these established cytokines, Th9 cells have also been suggested to produce IL-3 in an interaction with dendritic cells to enhance their longevity [64,65]. The effect of these cytokines in asthma and allergy is less well understood.
Th17 Cells
Similar to the Th2 and Th9 subtypes, the Th17 subtype is also subject to the control through the activity of various transcription factors and cytokines. Most crucial among these are IL-6 and TGF-β, which are can combine to stimulate activation of the lineage-defining transcription factor RORγt [66-68]. Since the internal regulation mechanisms of Th17 cells have been the subject of many in-depth reviews, we will only add here that current definitions and understandings of Th17 cells may change significantly with further understanding of T helper plasticity, as a certain percentage of Tfh have been found to be highly RORγt expressing [69]. Interestingly, an alternative polarization combination of IL-6, IL-1b, and IL-23 may also generate a more pathogenic strain of Th17 cells [70]. As with Th2 and Th9 cells, the cytokine environment in asthma clearly allows for the differentiation of Th17 cells. Several studies have also confirmed that substantial amounts of Th17 cells are present in asthma and chronic inflammatory conditions [71].
IL-17, like IL-9, is generally a pro-inflammatory cytokine. 5 different IL-17 receptor (IL-17R) proteins have been identified, which are expressed across a wide variety of tissues, including epithelial and immune cells. IL-17 signaling promotes NF-kb activation and the associated inflammation in several ways, and may also enhance pro-inflammatory cell survival [72,73]. In particular, IL-17 can induce production of IL-6 in a positive feedback of sorts [74,75]. IL-17 can also stimulate increased expression of inducible nitric oxide synthase (iNOS), particularly in macrophages, leading to production of the reactive oxygen species NO [76-78]. This latter result is a negative feedback loop that can control Th17 activity, since NO has been shown to inhibit the Th17 subtype [79]. Of additional interest in asthma is the fact that IL-17 expression is significantly elevated in the local airway during acute responses, as well as in general allergic reactions [80]. These effects serve as potent complements to those driven by Th9, and may potentially arrive at the site at the same time as Th9, if not earlier. Further study of the chronology will be required for a more complete mechanistic understanding on a multi-cellular level.
Treatments
Current treatment methods for asthma can be classified into two general categories-long-term controlling medications that seek to suppress chronic inflammation, and short-term rescue medications that act rapidly in the event of an attack. While some of the medications used to treat asthma may “overlap” and be applied for both rescue and control, it is nonetheless clear that they are to be distinct. Short-term rescue medication is generally given at a far higher dose than control medication, with the need to act rapidly and effectively. Specificity, though desirable, may be sacrificed somewhat in such emergency situations. Long-term control medications, on the other hand, are generally given at much lower doses and maintained for years. As such, increased specificity for long-term control would be extremely beneficial. There is also hope that long-term control can eventually prove to become a cure, if it can permanently recalibrate the asthmatic response.
The standard short-term rescue medications are short-acting beta-adrenergic agonists (beta-agonists) such as epinephrine that relax airway muscles, and systemic corticosteroids that serve as broad immunosuppresants to reduce inflammation [81]. Beta-agonists act on beta adrenoreceptors on airway tissue to drive the activation of adenylate cyclase, starting a chain reaction that leads to smooth muscle relaxation [82-84]. While generally considered safe, they have also been suggested to exacerbate the condition in some circumstances [85]. Corticosteroids, on the other hand, act more broadly, and impact the phospholipid regulation in all cells. However, long-term use of corticosteroids may lead to a host of side effects, including obesity, which may undermine long-term control [86]. It may also lead to other detrimental effects such as hypertension and other metabolic deregulation [87]. Furthermore, studies have suggested that corticosteroids may actually be significantly resisted by at least Th17 cells when used to treat asthma [88,89]. While treatments such as a corticosteroid and statin combination have been proposed to overcome some of those issues, it is unclear if it would be useful as therapy, with the issue being currently in controversy [90-93]. It may also be that the high dosages at which rescue medications are used render their specificity to be negligible, while the lower dosages at which they are used as long-term controls makes those effects more visible.
Several different drugs have been under development in recent years to provide more specific treatment options for chronic asthma. Many of them seek to directly target T helper cell function. These anti-T helper drugs generally fall into three types; anti-cytokine antibodies, cytokine receptor agonists, and cytokine receptor mimics. Designed to disrupt the inflammation machinery, these drugs have the additional potential of progressing towards an actual cure by clearing out the excess population of pro-inflammatory T helpers. (It should be noted that such a clearing out would need to be managed with caution, given that pro-inflammatory T helpers are still vital to proper immune function.) Many of the drugs showed great efficacy in murine models. Unfortunately however, such successes have yet to be replicated sufficiently in clinical trials.
The earliest of these more selective drugs was a recombinant human IL-4 receptor that showed promise in early phases, but which failed to prove true clinical efficacy in further trials [94]. A subsequently developed humanized anti-IL-4 antibody similarly failed to be effective at suppressing Th2 cells in trials, although a more recent version has been demonstrated to be more effective, especially in reducing eosinophil counts [95-99]. A humanized monoclonal anti-IL-9 antibody has been successfully generated and has reached phase 2a in clinical trials, but has not been found to lead to significant improvements in patient condition when combined with existing asthma controller medications [100,101]. It is unclear if other specific antibodies or agonists targeting IL-9 or IL-9R have been generated. Humanized anti-IL-17 antibodies and IL-17R agonists have been generated by several pharmaceutical companies and have been tested on a wide spectrum of autoimmune disorders [102-105]. Encouragingly, both have been shown to be effective in treating psoriasis, a skin disorder sometimes associated with asthma [106,107]. Unfortunately, the use of IL-17R agonist did not lead to significantly improved outcomes in a phase 2 clinical trial for asthma [108]. In addition, both the IL-17R agonist and the anti-IL-17 antibody have been shown to be ineffective in treating ulcerative colitis, a disorder that features similar T helper interplay to asthma [109]. Further testing may resolve the exact efficacy of these anti-IL-17 medications.
Other anti-cytokine drugs have also been developed to target mast cells and macrophages, with varying degrees of success. Anti-IL-5 medication is currently in testing, and has shown particularly efficacy against eosinophils [110]. Histamine receptor agonists are now in common use, and mast cell stabilizers have also been developed [111-114]. However, these medications primarily function in alleviating the short-term acute response, and may not be very suitable for longer term use due to side effects such as drowsiness. Leukotriene receptor agonists have also seen some success [115]. Anti-TNF-a drugs targeting persistent macrophages have also been shown to be unsuitable due to a low response rate [116]. Targeting of several other surface proteins, such as OX40 and VLA-4 has been shown to be quite limited in effect [117,118]. Intriguingly, clinical trial of an anti-TSLP antibody that would inhibit both Th2 and Th9 development and memory revealed significant improvements in indices of airway inflammation and bronchodilation [119]. While the Th2 targeting treatment is perhaps the most promising now given its stage in clinical trial, it may still require significant further refinements. Overall, it must be concluded that anti-cytokine therapy may not be the most optimal strategy, given the low rate of success.
As such, the development of other compounds for treating allergic asthma is still urgently needed. Genotyping may help in this process significantly, as some subsets with genetic differences about a cytokine that predispose to asthma may be more receptive to treatments targeting that cytokine. Other possible targets such as transcription factors and other polarization factors may also be of use for therapy. While gene therapy has historically encountered great difficulty in translating to cures, the recent European Commission approval of alipogene tiparvovec has indicated a renewed interest in such techniques. microRNA sequences that have been linked with asthma may also be possible therapeutic targets with higher specificity [120].
The recent focus in immunology on the influence of metabolic factors in immune behavior has suggested that metabolism-based selection compounds may prove to be quite effective in treating autoimmune conditions. After all, conditions that disrupt normal metabolism, such as obesity, have been clinically correlated with increased asthma severity. Different T helper cell types have some significant variations in metabolic requirements; perhaps the most studied of these differences is the fact that T effector phenotypes including Th2, Th9, and Th17 all require higher energy during polarization than Tregs [121-123]. More interestingly however, different T helper phenotypes also have varied requirements for specific nutrients/metabolites [124,125]. Studies conducted on Th17 cells have demonstrated that the subtype is favorably polarized in the presence of succinate and brief hypoxia, in contrast to other subtypes [126,127]. In addition, Th17 cells have been shown to be more strongly affected by amino acid starvation than other T helper subtypes [128,129]. While Th17 cells are obviously not equivalent to Th9 cells (as evidenced by their sharply different responses to NO), small molecule compounds that target cellular metabolism may nonetheless prove to be quite useful in treating asthma.
Interestingly, recent studies have suggested that some perceived “broad suppressants” may actually be having selective and specific effects against the cells being acted upon, particularly at lower dosages. One study has found that the chemotherapy drug 5-fluorouracil, an anti-metabolite, may have specific effects against some T helper phenotypes while leaving others unaffected, and that it may induce these effects without inducing widespread apoptosis as feared (private communication). A similar mechanism could be underpinning the successful use of a similar anti-metabolite, methotrexate, in its off-label application against atopic dermatitis [130]. It would not be so fanciful a notion to consider that such medications could also be relevant in treating asthma. Some of these medications are already in use in treating small-cell lung cancer, among other lung conditions [131]. Studies on the drug tolerance of these medications by the other cells along the airway as compared to their tolerance in the gut could further demonstrate their feasibility.
Dose-based analysis may also reveal more specific effects for other non-metabolic medications as well. For instance, beta-agonist mediated activation of adenylate cyclase could promote the breakdown of intracellular ATP, and consequently drive depletion of extracellular ATP, which would then influence T helper behavior, possibly biasing against Th17 proliferation [132,133]. Such a phenomenon may be a reason for why low-dose use of long-term beta-agonists can help improve management when used in conjunction with corticosteroids. Similarly, recent work has shown that inhibition of a broad-acting positive effector of transcription, BRD4, can be inhibited to selective effects against some T helper types (unpublished data in addition to references) [134,135]. Overall then, it may be useful to apply these other compounds in conjunction or slightly after the standard short-term controllers to strongly regulate the T helper cell activity that occurs following the immediate inflammatory event, to prevent airway reconstruction that occurs later on.
Conclusion
A complex condition featuring the interplay of many different transcription factors across different cell types, asthma remains a difficult condition to over the long-term. The current generation of non-specifically (or at non-intentionally specific) medications being used have significant drawbacks that impair quality of life, but the next generation of more specific anti-cytokine treatments have not been able to pass clinical trials. Despite the brevity of their existence, T helper cells polarized to Th2, Th9, and Th17 subtypes play major roles in controlling inflammation events, and may influence immune activity even after the cells have passed. Additional memory versions of these cells can also induce stronger responses during subsequent challenges along the road towards deleterious airway reconstruction. As such, specific inhibition of these subtypes may be critical towards treating asthma and progressing towards a cure. While many of the specific anti-cytokine therapies attempted so far have not been successful, anti-metabolic and anti-memory therapies have shown some promise (a summary of the specificity certain medications may have is presented in Table 1).
![]()
Table 1: Table of the cell types each treatment has been shown to directly affect
View Table 1
References
-
Winer RA, Qin X, Harrington T, Moorman J, Zahran H (2012) Asthma incidence among children and adults: findings from the Behavioral Risk Factor Surveillance system asthma call-back survey-United States, 2006-2008. J Asthma 49: 16-22.
-
ISAAC (2011) Global Asthma Report 2011. Paris, France: The International Union Against Tuberculosis and Lung Disease.
-
Wheatley LM, Togias A (2015) Clinical practice. Allergic rhinitis. N Engl J Med 372: 456-463.
-
Delgado J, Barranco P, Quirce S (2008) Obesity and asthma. J Investig Allergol Clin Immunol 18: 420-425.
-
Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, et al. (2014) Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med 20: 54-61.
-
Wang Q, Du J, Zhu J, Yang X, Zhou B (2015) Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol 135: 781-791.
-
Mukherjee S, Rasky AJ, Lundy PA, Kittan NA, Kunkel SL et al. (2014) STAT5-induced lunatic fringe during Th2 development alters delta-like 4-mediated Th2 cytokine production in respiratory syncytial virus-exacerbated airway allergic disease. J Immunol 192: 996-1003.
-
Ashino S, Takeda K, Li H, Taylor V, Joetham A, et al. (2014) Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J Allergy Clin Immunol 133: 1162-1174.
-
Zhang DH, Cohn L, Ray P, Bottomly K, Ray A (1997) Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 272: 21597-21603.
-
Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587-596.
-
Eifan AO, Furukido K, Dumitru A, Jacobson MR, Schmidt-Weber C, et al. (2012) Reduced T-bet in addition to enhanced STAT6 and GATA3 expressing T cells contribute to human allergen-induced late responses. Clin Exp Allergy 42: 891-900.
-
Yagi R, Junttila IS, Wei G, Urban JF Jr, Zhao K, et al. (2010) The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity 32: 507-517.
-
Hwang SS, Kim YU, Lee S, Jang SW, Kim MK, et al. (2013) Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proc Natl Acad Sci U S A 110: 276-281.
-
Mari N, Hercor M, Denanglaire S, Leo O, Andris F (2013) The capacity of Th2 lymphocytes to deliver B-cell help requires expression of the transcription factor STAT3. Eur J Immunol 43: 1489-1498.
-
Lebman DA, Coffman RL (1988) Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med 168: 853-862.
-
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2014) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine.
-
Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, et al. (2013) Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy 68: 55-63.
-
Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, et al. (2008) IL-4 inhibits TGF-βeta-induced Foxp3+ T cells and, together with TGF-βeta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 9: 1347-1355.
-
Wang Q, Du J, Zhu J, Yang X, Zhou B (2015) Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol 135: 781-791.
-
Anuradha R, George PJ, Hanna LE, Chandrasekaran V, Kumaran, P, et al. (2013) IL-4-, TGF-ß-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J Immunol 191: 2466-2473.
-
Angkasekwinai P, Srimanote P, Wang YH, Pootong A, Sakolvaree Y, et al. (2013) Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect Immun 81: 3731-3741.
-
Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM (2012) Activin A and TGF- ß promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol 129: 1000-1010.
-
Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, et al. (2010) Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33: 192-202.
-
Vasanthakumar R, Mohan V, Anand G, Deepa M, Babu S, et al. (2015) Serum IL-9, IL-17, and TGF- ß levels in subjects with diabetic kidney disease (CURES-134). Cytokine 72: 109-112.
-
McNamara PS, Flanagan BF, Baldwin LM, Newland P, Hart CA, et al. (2004) Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 363: 1031-1037.
-
Lin JY, Chen JS, Hsu CJ, Miaw SC, Liu CY, et al. (2012) Epicutaneous sensitization with protein antigen induces Th9 cells. J Invest Dermatol 132: 739-741.
-
Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, et al. (2014) Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol 175: 25-31.
-
Pawankar R, Hayashi M, Yamanishi S, Igarashi T (2015) The paradigm of cytokine networks in allergic airway inflammation. Curr Opin Allergy Clin Immunol 15: 41-48.
-
Niedbala W, Besnard AG, Nascimento DC, Donate PB, Sonego F1, et al. (2014) Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nat Commun 5: 4575.
-
Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, et al. (2010) Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol 185: 6795-6801.
-
Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, et al. (2014) Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 6: 219ra8.
-
Kaplan MH (2013) Th9 cells: differentiation and disease. Immunol Rev 252: 104-115.
-
Chen N, Lu K, Li P, Lv X, Wang X (2014) Overexpression of IL-9 induced by STAT6 activation promotes the pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp Pathol 7: 2319-2323.
-
Wang A, Pan D, Lee YH, Martinez GJ, Feng XH, et al. (2013) Cutting edge: Smad2 and Smad4 regulate TGF- ß-mediated Il9 gene expression via EZH2 displacement. J Immunol 191: 4908-4912.
-
Li D, Chen D, Zhang X, Wang H, Song Z, et al. (2015) c-Jun N-terminal kinase and Akt signalling pathways regulating tumour necrosis factor-a-induced interleukin-32 expression in human lung fibroblasts: implications in airway inflammation. Immunology 144: 282-290.
-
Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, et al. (2012) STAT6-dependent regulation of Th9 development. J Immunol 188: 968-975.
-
Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, et al. (2010) Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33: 192-202.
-
Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A (2012) Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood 119: 4665-4674.
-
Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, et al. (2014) TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 15: 676-686.
-
Végran F, Berger H, Boidot R, Mignot G, Bruchard M, et al. (2014) The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol 15: 758-766.
-
Goswami R, Kaplan MH (2011) A brief history of IL-9. J Immunol 186: 3283-3288.
-
Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, et al. (2010) Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33: 192-202.
-
Nalleweg N, Chiriac MT, Podstawa E, Lehmann C, Rau TT, et al. (2015) IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 64: 743-755.
-
Leng RX, Pan HF, Ye DQ, Xu Y (2012) Potential roles of IL-9 in the pathogenesis of systemic lupus erythematosus. Am J Clin Exp Immunol 1: 28-32.
-
Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, et al. (2009) IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med 206: 1653-1660.
-
Blankenhaus B, Reitz M, Brenz Y, Eschbach ML, Hartmann W, et al. (2014) Foxp3? regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathog 10: e1003913.
-
Licona-Limón P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limón I, et al. (2013) Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity 39: 744-757.
-
Wang YH, Hogan SP, Fulkerson PC, Abonia JP, Rothenberg ME (2013) Expanding the paradigm of eosinophilic esophagitis: mast cells and IL-9. J Allergy Clin Immunol 131: 1583-1585.
-
Dragon S, Takhar MK, Shan L, Hayglass KT, Simons FE, et al. (2009) T(H)2 cytokines modulate the IL-9R expression on human neutrophils. Biochem Biophys Res Commun 384: 167-172.
-
Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, et al. (2003) IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol 170: 3461-3467.
-
Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, et al. (2007) Expression of IL-9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 120: 1208-1215.
-
Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM (1993) Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology 79: 146-151.
-
Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, et al. (2007) Expression of IL-9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 120: 1208-1215.
-
Baraldo S, Faffe DS, Moore PE, Whitehead T, McKenna M, et al. (2003) Interleukin-9 influences chemokine release in airway smooth muscle: role of ERK. Am J Physiol Lung Cell Mol Physiol 284: L1093-1102.
-
Pilette C, Ouadrhiri Y, Van Snick J, Renauld JC, Staquet P, et al. (2002) Oxidative burst in lipopolysaccharide-activated human alveolar macrophages is inhibited by interleukin-9. Eur Respir J 20: 1198-1205
-
Tsukadaira A, Okubo Y, Koyama S, Sato E, Nagase H, et al. (2002) Human bronchial epithelium expresses interleukin-9 receptors and releases neutrophil chemotactic factor. Exp Lung Res 28: 123-139.
-
Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, et al. (2013) IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med 210: 2951-2965.
-
Gong Y, Huang L, Cheng W, Li X, Lu J, et al. (2014) Roles of interleukin-9 in the growth and cholecystokinin-induced intracellular calcium signaling of cultured interstitial cells of Cajal. PLoS One 9: e95898.
-
Ng CT, Oldstone MB (2014) IL-10: achieving balance during persistent viral infection. Curr Top Microbiol Immunol 380: 129-144.
-
Végran F, Berger H, Boidot R, Mignot G, Bruchard M, et al. (2014) The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol 15: 758-766.
-
Lu Y, Hong S, Li H, Park J, Hong B, et al. (2012) Th9 cells promote antitumor immune responses in vivo. J Clin Invest 122: 4160-4171.
-
Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, et al. (2014) Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 345: 1058-1062.
-
Kwok SK, Lee J, Yu D, Kang KY, Cho M, et al. (2015) A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat Rev Rheumatol .
-
Park J, Li H, Zhang M, Lu Y, Hong B, et al. (2014) Murine Th9 cells promote the survival of myeloid dendritic cells in cancer immunotherapy. Cancer Immunol Immunother 63: 835-845.
-
Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, Weiner HL (2012) IFN-? limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. J Immunol 189: 5277-5283.
-
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, et al. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121-1133.
-
Kimura A, Naka T, Kishimoto T (2007) IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A 104: 12099-12104.
-
Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, et al. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28: 29-39.
-
Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, et al. (2014) The cytokine TGF-ß co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 15: 856-865.
-
Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, et al. (2012) Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13: 991-999.
-
Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, et al. (2008) Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 180: 7423-7430.
-
Yang S, Wang Y, Mei K, Zhang S, Sun X, et al. (2015) Tumor Necrosis Factor Receptor 2 (TNFR2)•Interleukin-17 Receptor D (IL-17RD) Heteromerization Reveals a Novel Mechanism for NF- ?B Activation. J Biol Chem 290: 861-871.
-
Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, et al. (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 160: 3513-3521.
-
Varelias A, Gartlan KH, Kreijveld E, Olver SD, Lor M, et al. (2015) Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 125: 2435-2444.
-
Feng JS, Yang Z, Zhu YZ, Liu Z, Guo CC, et al. (2014) Serum IL-17 and IL-6 increased accompany with TGF-ß and IL-13 respectively in ulcerative colitis patients. Int J Clin Exp Med 7: 5498-5504.
-
Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, et al. (2001) Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol 119: 183-191.
-
Mori D, Watanabe N, Kaminuma O, Murata T, Hiroi T, et al. (2014) IL-17A induces hypo-contraction of intestinal smooth muscle via induction of iNOS in muscularis macrophages. J Pharmacol Sci 125: 394-405.
-
Van Bezooijen RL, Papapoulos SE, Löwik CW (2001) Effect of interleukin-17 on nitric oxide production and osteoclastic bone resorption: is there dependency on nuclear factor-kappaB and receptor activator of nuclear factor kappaB (RANK)/RANK ligand signaling? Bone 28: 378-386.
-
Jianjun Yang, Zhang R, Lu G, Shen Y, Peng L, et al. (2013) T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation. J Exp Med 210: 1447-1462.
-
Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, et al. (2013) Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy 43: 1018-1026.
-
Chung KF, Caramori G, Adcock IM (2009) Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol 65: 853-871.
-
Molinoff PB (1984) Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 28: 1-15.
-
Johnson M (2006) Molecular mechanisms of beta (2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol 117: 18-24.
-
Morgan SJ, Deshpande DA, Tiegs BC, Misior AM, Yan H, et al. (2014) ß-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J Biol Chem 289: 23065-23074.
-
Chiarella SE, Budinger GR, Mutlu GM3 (2015) ß2- Agonist therapy may contribute to the air pollution and IL-6-associated risk of developing severe asthma with dual-positive TH2/TH17 cells. J Allergy Clin Immunol 135: 290-291.
-
Sivapalan P, Diamant Z, Ulrik CS (2015) Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med 21: 80-85.
-
Hjern F, Mahmood MW, Abraham-Nordling M, Wolk A, Hakansson N (2015) Cohort study of corticosteroid use and risk of hospital admission for diverticular disease. Br J Surg 102: 119-124.
-
McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, et al. (2008) TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 181: 4089-4097.
-
Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, et al. (2014) Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 211: 89-104.
-
Ingebrigtsen TS, Marott JL, Nordestgaard BG, Lange P, Hallas J, et al. (2015) Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax 70: 33-40.
-
Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, et al. (2013) Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am J Respir Crit Care Med 188: 1076-1082.
-
Zein JG (2013) Should all patients with asthma receive statins? Am J Respir Crit Care Med 188: 1177-1178.
-
Tse SM, Charland SL, Stanek E, Herrera V, Goldfarb S, et al. (2014) Statin use in asthmatics on inhaled corticosteroids is associated with decreased risk of emergency department visits. Curr Med Res Opin 30: 685-693.
-
Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, et al. (1999) Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med 160: 1816-1823.
-
Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, et al. (2002) Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol 130: 93-100.
-
Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M (2007) Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370: 1422-1431.
-
Slager RE, Otulana BA, Hawkins GA, Yen YP, Peters SP, et al. (2012) IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor a antagonist. J Allergy Clin Immunol 130: 516-522.
-
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, et al. (2013) Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 368: 2455-2466.
-
Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, et al. (2014) Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 371: 130-139.
-
Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, et al. (2011) Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med 11: 14.
-
Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, et al. (2013) A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res 14: 93.
-
Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, et al. (2014) Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol 41: 1039-1046.
-
Abate MV, Sandrin C, Pastore S (2014) Secukinumab for ankylosing spondylitis. Lancet 383: 780.
-
Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, et al. (2014) One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol 41: 414-421.
-
Papp K, Menter A, Strober B, Kricorian G, Thompson EH, et al. (2015) Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol 72: 436-439.
-
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, et al. (2014) Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 371: 326-338.
-
Osamu N, Hirotaka N, Koji S, Kenji T (2014) Clinical pharmacology of the anti-IL-17 receptor antibody brodalumab (KHK4827) in Japanese normal healthy volunteers and Japanese subjects with moderate to severe psoriasis: a randomized, dose-escalation, placebo-controlled study. J Dermatol Sci 75: 201-204.
-
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, et al. (2013) Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188: 1294-1302.
-
Kaser A (2014) Not all monoclonals are created equal - lessons from failed drug trials in Crohn's disease. Best Pract Res Clin Gastroenterol 28: 437-449.
-
Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, et al. (2013) Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 131: 1576-1582.
-
Ang DC, Hilligoss J, Stump T (2014) Mast Cell Stabilizer (Ketotifen) in Fibromyalgia: Phase 1 Randomized Controlled Clinical Trial. Clin J Pain.
-
MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, et al. (2014) The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35. Mol Pharmacol 85: 91-104.
-
Capron M, Loiseau S, Papin JP, Robertson S, Capron A (1998) Inhibitory effects of lodoxamide on eosinophil activation. Int Arch Allergy Immunol 116: 140-146.
-
Matsumoto K, Aizawa H, Fukuyama S, Yoshida M, Komori M, et al. (2013) Low-dose salbutamol suppresses airway responsiveness to histamine but not methacholine in subjects with asthma. Respir Investig 51: 158-165.
-
Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, et al. (2001) Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 108: 48.
-
Thomas PS, Heywood G (2002) Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax 57: 774-778.
-
Gauvreau GM, Boulet LP, Cockcroft DW, FitzGerald JM, Mayers I, et al. (2014) OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy 44: 29-37.
-
Norris V, Choong L, Tran D, Corden Z, Boyce M, et al. (2005) Effect of IVL745, a VLA-4 antagonist, on allergen-induced bronchoconstriction in patients with asthma. J Allergy Clin Immunol 116: 761-767.
-
Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, et al. (2014) Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 370: 2102-2110.
-
Simpson LJ, Patel S, Bhakta NR, Choy DF, Brightbill HD, et al. (2014) A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol 15: 1162-1170.
-
Barbi J, Pardoll D, Pan F (2013) Metabolic control of the Treg/Th17 axis. Immunol Rev 252: 52-77.
-
Ghesquière B, Wong BW, Kuchnio A, Carmeliet P (2014) Metabolism of stromal and immune cells in health and disease. Nature 511: 167-176.
-
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, et al. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299-3303.
-
Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, et al. (2014) Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40: 692-705.
-
Byersdorfer CA, Tkachev V, Opipari AW, Goodell S, Swanson J, et al. (2013) Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood 122: 3230-3237.
-
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, et al. (2013) Succinate is an inflammatory signal that induces IL-1ß through HIF-1a. Nature 496: 238-242.
-
Shi LZ, Wang R, Huang G, Vogel P, Neale G, et al. (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367-1376.
-
Carlson TJ, Pellerin A, Djuretic IM, Trivigno C, Koralov SB, et al. (2014) Halofuginone-induced amino acid starvation regulates Stat3-dependent Th17 effector function and reduces established autoimmune inflammation. J Immunol 192: 2167-2176.
-
Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, et al. (2009) Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324: 1334-1338.
-
Zoller L, Ramon M, Bergman R (2008) Low dose methotrexate therapy is effective in late-onset atopic dermatitis and idiopathic eczema. Isr Med Assoc J 10: 413-414.
-
Zhao JG, Ren KM, Tang J (2014) Overcoming 5-Fu resistance in human non-small cell lung cancer cells by the combination of 5-Fu and cisplatin through the inhibition of glucose metabolism. Tumour Biol 35: 12305-12315.
-
Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vázquez G, et al. (2015) The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42: 41-54.
-
Killeen ME, Ferris L, Kupetsky EA, Falo L Jr, Mathers AR (2013) Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol 190: 4324-4336.
-
Mele DA, Salmeron A, Ghosh S, Huang HR, Bryant BM, et al. (2013) BET bromodomain inhibition suppresses TH17-mediated pathology. J Exp Med 210: 2181-2190.
-
Bandukwala HS, Gagnon J, Togher S, Greenbaum JA, Lamperti ED, et al. (2012) Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc Natl Acad Sci U S A 109: 14532-14537.





