Clinical Medical
Reviews and Case Reports
Progressive Course of Infective Endocarditis Complicated with Basilar Artery Aneurysm: Serial Cranial Magnetic Resonance Imaging Findings
Neslin Sahin*, Aynur Solak, Berhan Genc and UgurKulu
Department of Radiology, Sifa University School of Medicine, Turkey
*Corresponding author: Neslin Sahin, Department of Radiology, Sifa University School of Medicine, Fevzipasa Boulevard No. 172/2, 35240 Basmane Izmir, Turkey, Tel: +90-232-343-44-45, Fax : +90-232-343-56-56, E-mail: neslinshn@gmail.com
Clin Med Rev Case Rep, CMRCR-2-033, (Volume 2, Issue 5), Case report; ISSN: 2378-3656
Received: February 16, 2015 | Accepted: May 25, 2015 | Published: May 27, 2015
Citation: Sahin N, Solak A, Genc B, Kulu U (2015) Progressive Course of Infective Endocarditis Complicated with Basilar Artery Aneurysm: Serial Cranial Magnetic Resonance Imaging Findings. Clin Med Rev Case Rep 2:033. 10.23937/2378-3656/1410033
Copyright: © 2015 Sahin N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We report a case of a 31-year-old man with a basilar artery aneurysm and associated different imaging findings caused by a septic embolus originating from infective mitral valve endocarditis. Cerebrovascular complications (CVC) are well-known causes of morbidity and mortality in patients with infective endocarditis (IE). Patients with IE may present with different types of neuroradiological findings alone or in combination and thus have a worse prognosis than patients without CVCs. Infectious intracranial aneurysms are rare, but potentially fatal complications of IE. However, diagnosis of IE and associated complications can be difficult due to nonspecific symptoms. In this paper, the role of neurovascular imaging for a diagnostic workup of the CVCs of IE is discussed.
Keywords
Aneurysm, Cerebral infarction, Infective endocarditis, Magnetic resonance imaging, Septic embolism
Introduction
Cerebrovascular complications (CVC) are well-known causes of morbidity and mortality with an incidence of 20%-40% in patients with infective endocarditis (IE) [1]. However, diagnosis of IE and associated complications can be difficult due to nonspecific symptoms [2]. Furthermore, symptomatic neurologic complications of IE are frequent, but asymptomatic cerebral embolismdiagnosed by magnetic resonance imaging (MRI) occurs in many more patients [1,3].
Intracranial infectious aneurysms (IIA; commonly referred to as mycotic aneurysms) are detected in 2%-4% of patients with IE [4]. Compared to berry aneurysms, IIAs are usually multiple, particularly at peripheral branch points, and they are more prone to rupture with poorly defined necks, and thus, have a high associated mortality [5,6]. In this paper, a patient reported with a basilar artery (BA) aneurysm and associated different imaging findings caused by a septic embolus originating from infective mitral valve endocarditis. This difficult case is used to discuss the role of neurovascular imaging for a diagnostic workup of the cerebrovascular complications of IE.
Case Report
A 31-year-old male with a history of mitral valve disease was presented to the emergency department with severe headaches, dizziness, nausea, vomiting, and disequilibrium. An unenhanced cranial CT performed at another facility for left arm numbness, in addition to these similar but less severe complaints, was within the normal limits of two days previously. His temperature was normal. Systolic and diastolic murmurs were determined on physical examination. No neurologic deficits were present.
The chest X-ray study and electrocardiogram were normal. An echocardiogram showed a 10x12mm mitral valve vegetation with severe mitral regurgitation. Laboratory tests with positive C-reactive protein, elevated erythrocyte sedimentation rate, and polymorphonuclear leukocytosis were supportive of IE. The Cranial MRI showed a 16mm hyperintense lesion with a central hypointense area with peripheral edema on T2-weighted, revealing minimal peripheral contrast enhancement (bull's-eye-like lesion) that was hypointense on susceptibility-weighted imaging (SWI) in the right occipital lobe (Figure 1a,1b). In addition, the MRI revealed a 12mm early subacute hemorrhage in the right frontal lobe with microhemorrhages (mHs) in the bilateral cerebral and cerebellar hemispheres predominantly involving the cortices (Figure 1C). The blood culture was positive for Streptococcus alactolyticus and antibiotic therapy was started. The cardiothoracic surgery team suggested a follow up for reassessment after medical treatment. On hospital day 7, the MRI demonstrated additional right parieto occipital hemorrhage. On hospital day 12, the cranial MRI established a new BA aneurysm without a hemorrhage and new hyperintense ischemic changes in the bilateral thalami and mesencephalon on T2-weighted images (Figure 1d-1f). The MR angiography (MRA) and CT angiography (CTA), performed to better characterize the aneurysm, showed a multilobulated fusiform BA tip aneurysm involving the origins of both posterior cerebral arteries (PCA) measuring 12x9mm and associated with diffuse vasospasm in the distal BA (Figure2a-2c). On hospital day 24, the aneurysm was enlarged (21x17mm) according to the control cranial MRI (Figure 2d). One day later, the patient's condition deteriorated and ptosis of the left eye developed. Cerebral digital subtraction angiography (DSA) confirmed a fusiform BA aneurysm with occlusion of the right PCA.
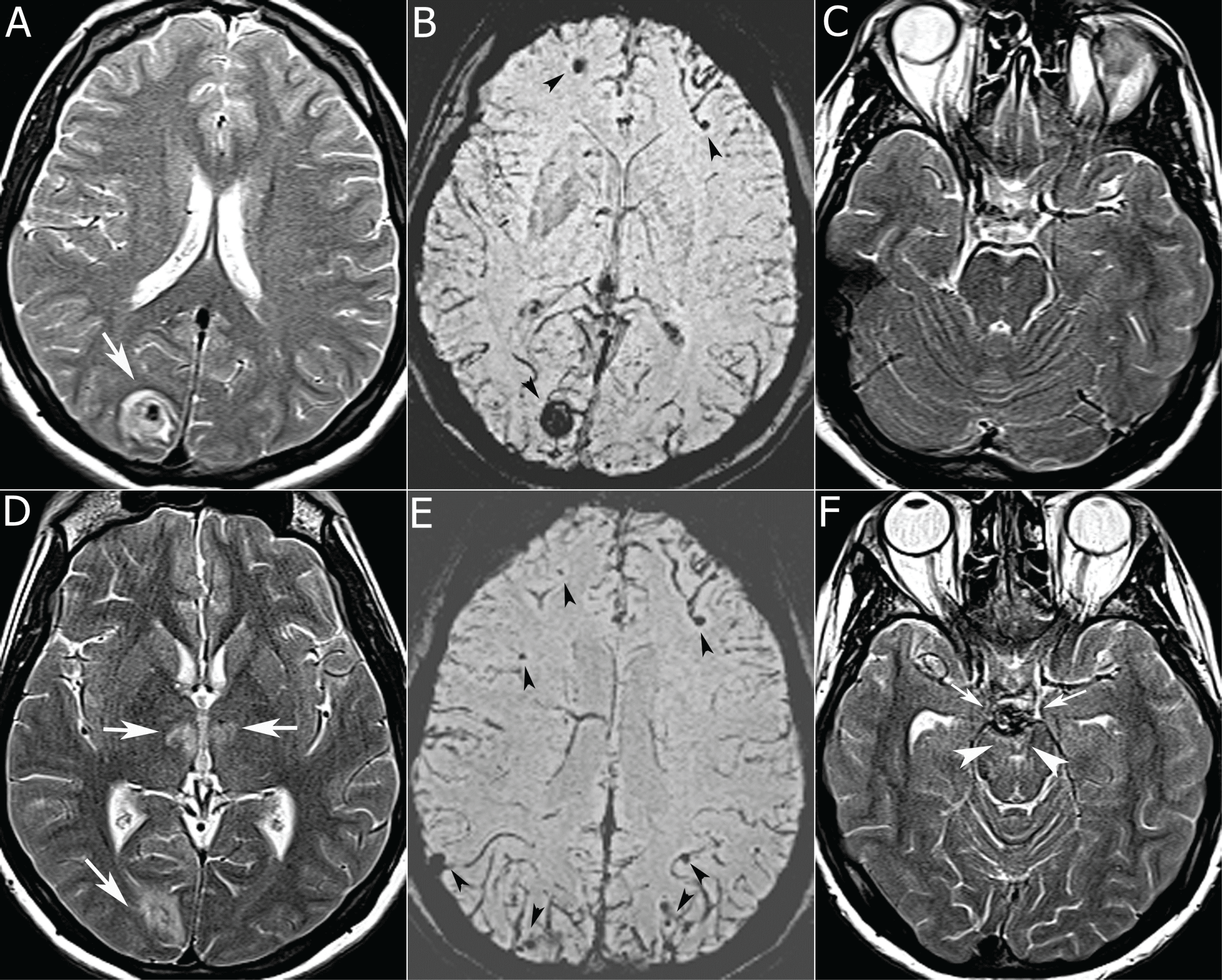
.
Figure 1: T2-weighted axial image (a) shows a bull's-eye-like lesion with a central hypointense area (arrow), hypointense on SWI (b) as microhemorrhages (mHs, black arrowheads). On day 12, T2-weighted axial image (d) demonstrates new hyperintense ischemic signal changes in thalami (white arrows) in addition to bull's eye lesion (white arrow). SWI (e) reveals more mHs predominantly in the cortices (black arrowheads). T2- weighted axial image (f) also shows a new basilar artery aneurysm (small arrows) and new ischemic changes in mesencephalon (white arrowheads), not present in initial MRI (c).
View Figure 1
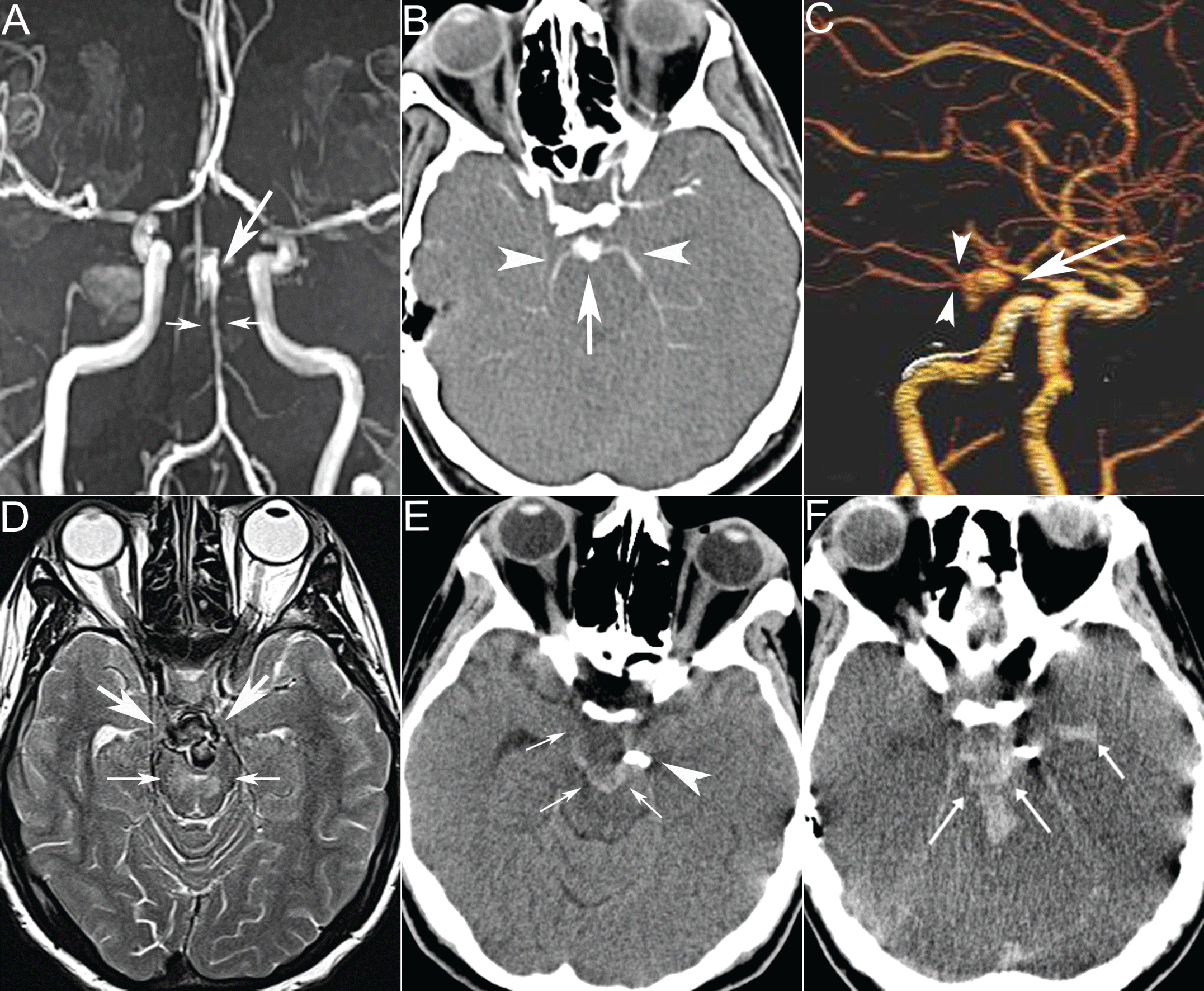
.
Figure 2: MR angiography (a) shows multilobulated fusiform basilar artery tip aneurysm (arrow) with vasospasm in distal basilar artery (small arrows). CT angiography (b, c), confirms aneurysm (arrow) involving the posterior cerebral arteries (arrowheads). On day 24, T2-weighted axial image (d) shows aneurysm enlargement (arrows) with more prominent ischemia (small arrows). Control CT (e) reveals a stent (arrowhead) across the neck of aneurysm (small arrows). 2 days after the procedure, CT shows aneurysmal rupture resulting in subarachnoid and intraventricular hemorrhage (arrows) and brain edema.
View Figure 2
The aneurysm was treated with a pipeline flow diverting stent placement. Endovascular treatment was successful; however the patient became unconscious after the procedure. No new findings were present on the cranial CT (Figure 2e). Two days after the procedure, a diffuse subarachnoid hemorrhage (SAH) developed from an aneurysmal rupture (Figure 2f) and the patient died.
Discussion
Cerebrovascular complications commonly occur in IE particularly in the setting of left-sided valvular disease related to cerebral embolization, even with appropriate diagnosis and therapy [1,3]. Besides, these complications have been described as the first symptom in up to 47% of the cases [3]. Ischemic stroke is the most common presentation whereas other manifestations-hemorrhagic stroke, intracranial hemorrhage or SAH, brain abscess, meningitis, cerebritis and infectious aneurysms-are less frequent [2,3].
A very high frequency of asymptomatic cerebral lesions were reported in many more patients (up to 71.5%) by MRIs reflecting the embolic potential of valvular vegetation [1,7]. In a study by Hess et al. the most frequent occult lesions were cerebral MHs, preferentially in cortical areas (62 patients) and acute ischemic lesions, mostly as multiple small watershed infarcts (40 patients) [1]. Eight patients had SAH, three brain micro abscesses, three small cortical hemorrhages, and three aneurysms. Cerebral mHs have been proposed as being indicative of the presence of a mycotic aneurysm as cerebral aneurysms were observed at the corresponding sites [8,9]. Kin et al. [8] did not visualize any aneurysm associated with mHs that was explained by the non detectability of these signals as aneurysms due to the small size or thrombosed aneurysm. Hess et al. [1] found mHs in more than half of the patients, but aneurysms in only 3% of the patients. However, asymptomatic patients were involved in their study and hemorrhagic lesions were infrequent.
Bull's-eye-like lesions, appearing as hyperintense lesions surrounding a central hypointense focus on T2-weighted and/or T2*-weighted imaging, were reported in four of a total 13 IE patients with neurological complications. The central hypointense focus suggested microbleeding, an infectious aneurysm or a bacterial clot in the vascular structure and the peripheral hyperintensity representing an inflammatory reaction [2].
Previous studies demonstrated that the detection of cerebral lesions affected diagnostic decisions in cases of suspected IE and contributed to therapeutic planning [1,7,10]. MRIs were found to be more sensitive than CTs in identifying both symptomatic lesions (100% and 81%, respectively) and additional occult lesions (50% and 23%, respectively) [10]. Therefore, an MRI with DWI and gradient-echo sequences were recommended for all patients with suspected IE to recognize cerebral lesions, including acute ischemic lesions and mHSs that cannot be diagnosed by CT or conventional MRI.
Our patient presented with cerebral mHs and bull's-eye-like lesions and subsequently developed IIA and secondary ischemia. The possibility of IIA development may be considered in patients presenting with mHs and bull's-eye-like lesions related to vascular vulnerability and cranial MRI with MRA may provide early diagnosis of IIA in such cases. Further detailed studies are needed to define the clinical significance of associated hemorrhages and mHs in the development of IIA.
IIAs are rare but potentially fatal neurovascular complications of IE, accounting for 0.7%-6.5% of all intracranial aneurysms [5,6]. Staphylococcus aureus and streptococcus species are the most common organisms; however the causative agent can be identified in only 30%-47% of the patients [4]. Septic emboli from friable cardiac vegetations typically lodge in multiple distal branches of the middle or less commonly posterior cerebral artery. These emboli may occlude vessels, cause multiple infarctions, or promote severe inflammation by spreading outward to the vessel wall and eventually resulting in aneurysm formation and enlargement. As IIAs are commonly asymptomatic and some of these cases could be resolved by medical treatment, the incidence of IIAs may be higher than as shown in the literature. Infected aneurysms are most commonly presented with symptoms related to intraparenchymal or subarachnoid hemorrhages, resulting either from necrotic arteritis or aneurysmal rupture. They are thin-walled with a poorly defined neck, and thus have a higher risk of rupture with fatal bleeding than berry aneurysms. However, the course of IIA and its timing and rate of rupture are unclear. The overall mortality of unruptured IIAs is 30%, while it is 80% in ruptured aneurysms [5]. In our case, IIA demonstrated typical imaging findings including change in size, arterial stenosis and occlusion adjacent to the aneurysm, and associated cerebral infarction [1]. Our patient was stable when the IIA was noticed and the change in level of consciousness developed with the enlargement of the aneurysm, without a rupture. In retrospective analysis of previous MR studies, no aneurysm was visible, but small associated IIAs cannot be excluded without an MRA or a CTA.
A non-contrast CT can be helpful in identifying subarachnoid and intracerebral hemorrhages, but may not be able to detect unruptured aneurysms, particularly small ones that can be identified by CTA, MRA, or DSA. Recent advances in multi detector CT imaging have increased the resolution of CTA with the advantages of a lower risk of neurologic deficits and a contrast load than DSA.A previous study demonstrated that CTA had a sensitivity of 83% and specificity of 93% in comparison to DSA [11]. However, as compared to the sensitivity of 95.3% for aneurysms >3mm, the sensitivity was 45% for aneurysms <3mm.MRA is 87% sensitive and 95% specific as compared to CTA with advantages of avoiding contrast load; however for aneurysms smaller than 3 mm, the sensitivity of an MRA decreases to 38% from 61% [12]. It may be beneficial to perform cerebrovascular imaging in all patients with endocarditis manifesting neurological deficits because of the strong association between endocarditis and IIA. DSA remains the gold standard for the diagnosis of intracranial aneurysm and should be performed if an aneurysm is clinically suspected even if the CTA and/or MRA studies are normal.
The management and treatment of IIAs are based on large case series due to the lack of randomised controlled trials with universally accepted guidelines in addition to the relative rarity of IIAs and the variability of clinical presentations. In a comprehensive literature review of 27 clinical series involving 287 patients with IIA, 62% had improved outcomes, 20% suffered a further neurological decline, 5% died before any intervention, and 12% died immediately after surgical or endovascular intervention [5]. The literature supports the combination of antimicrobial therapy for an underlying predisposing infection and surgical and/or endovascular approaches depending on the location and character of the aneurysm and the clinical status of the patient. Despite antibiotic treatment, the basilar IIA developed and became symptomatic with enlargement in a short time, and thus, the risk of an aneurysmal rupture and death was considered high with antibiotic treatment alone. Endovascular therapy, the only choice for treatment because of the localization of the aneurysm, was performed and still the patient died from an aneurysmal rupture after the procedure.
As un ruptured IIAs are known to resolve after medical treatment due to spontaneous thrombosis, at least six weeks of antibiotic therapy is recommended with close monitorization by serial angiography for resolution and improvement [13,14]. Ruptured IIAs with hematoma and/or increased intracranial pressure would require surgical therapy in addition to aneurysms involving an eloquent location, while endovascular therapy could be performed for aneurysms without mass effects and in non-eloquent territorities [6]. Previous studies demonstrated that endovascular treatment is potentially safer and more effective than open craniotomy if performed at a tertiary center [13,14].
Conclusion
Patients with IE may present with different types of neuro radiological findings alone or in combination and thus have a worse prognosis than patients without cerebrovascular complications. The case discussed in this paper demonstrates that IIA aneurysms are serious, but rarely considered as potentially fatal complications of IE and emphasizes the importance of the serial MRI for diagnosis. It should be noted that IIA may develop during the treatment of IE and this possibility should be considered with new neurological complaints and deficits despite treatment. Intracranial hemorrhage with a bull's-eye appearance associated with mHs may be the potential indicators for IIA and serial MRI with MRA may be helpful for those cases with suspected IIA.
References
-
Hess A, Klein I, Iung B, Lavallee P, Ilic-Habensus E, et al. (2013) Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol 34: 1579-1584.
-
Azuma A, Toyoda K, O'uchi T (2009) Brain magnetic resonance findings in infective endocarditis with neurological complications. Jpn J Radiol 27: 123-130.
-
Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, et al. (2000) Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med 160: 2781-2787.
-
Martindale JL, Hayden EM (2012) Neurologic complaints in a patient with infective endocarditis. J Emerg Med 43: e429-433.
-
Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, et al. (2010) Intracranial infectious aneurysms: a comprehensive review. Neurosurg Rev 33: 37-46.
-
Kannoth S, Thomas SV (2009) Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care 11: 120-129.
-
Duval X, Iung B, Klein I, Brochet E, Thabut G, et al. (2010) Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 152: 497-504, W175.
-
Kin H, Yoshioka K, Kawazoe K, Mukaida M, Kamada T, et al. (2013) Management of infectious endocarditis with mycotic aneurysm evaluated by brain magnetic resonance imaging. Eur J Cardiothorac Surg 44: 924-930.
-
Subramaniam S, Puetz V, Dzialowski I, Barber PA (2006) Cerebral microhemorrhages in a patient with mycotic aneurysm: relevance of T2-GRE imaging in SBE. Neurology 67: 1697.
-
Goulenok T, Klein I, Mazighi M, Messika-Zeitoun D, Alexandra JF, et al. (2013) Infective endocarditis with symptomatic cerebral complications: contribution of cerebral magnetic resonance imaging. Cerebrovasc Dis 35: 327-336.
-
Colen TW, Wang LC, Ghodke BV, Cohen WA, Hollingworth W, et al. (2007) Effectiveness of MDCT angiography for the detection of intracranial aneurysms in patients with nontraumatic subarachnoid hemorrhage. AJR Am J Roentgenol189: 898-903.
-
Goddard AJ, Tan G, Becker J (2005) Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: current status. Clin Radiol 60: 1221-1236.
-
Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, et al. (2002) Endovascular treatment of cerebral mycotic aneurysms. Radiology 222: 389-396.
-
Chun JY, Smith W, Halbach VV, Higashida RT, Wilson CB, et al. (2001) Current multimodality management of infectious intracranial aneurysms. Neurosurgery 48: 1203-1213.





