Clinical Medical
Reviews and Case Reports
Metastatic Gallbladder Cancer Masquerading as Primary Colon Malignancy
Zhifei Sun1*, Jonathan Galeotti2, Shannon McCall2 and Christopher R. Mantyh1
1Department of General Surgery, Duke University, USA
2Department of Pathology, Duke University, USA
*Corresponding author: Zhifei Sun, Duke University Medical Center, Box 2817, Durham, NC 27710, USA, Tel: 919-681-3816, Fax: 919-681-7934; E-mail: zhifei.sun@duke.edu
Clin Med Rev Case Rep, CMRCR-2-024, (Volume 2, Issue 3), Case Report; ISSN: 2378-3656
Received: December 16, 2014 | Accepted: March 25, 2015 | Published: March 28, 2015
Citation: Sun Z, Galeotti J, McCall S, Mantyh CR (2015) Metastatic Gallbladder Cancer Masquerading as Primary Colon Malignancy. Clin Med Rev Case Rep 2:024. 10.23937/2378-3656/1410024
Copyright: © 2015 Sun Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution,and reproduction in any medium, provided the original author and source are credited.
Abstract
We report a 69-year-old female with personal history of gallbladder cancer who was referred to our institution with a circumferential obstructing intra-luminal colonic mass that possessed pathological features common to both primary colorectal as well as gallbladder cancer, which posed a significant diagnostic and treatment challenge. This case reviews the method and rationale for arriving at the final diagnosis of gallbladder cancer metastasis and highlights the importance of modern molecular diagnostics as well as close communication between the pathologist and surgeon in its process.
Keywords
(Limit of five) Gallbladder cancer metastases, Immunohistochemistry markers
Introduction
Gallbladder Cancer (GBC) is a rare but highly fatal malignancy of the biliary tract, with most cases being found in patients undergoing simple cholecystectomy for biliary stone disease [1]. Management of GBC is mainly surgical with “curative intent” for early stage (T1/T2 without nodal involvement) tumors; but even with radical resection, recurrence is high [2]. From the experience of Jarnagin et al. from Memorial Sloan Kettering from 1990-2001, among 80 patients with GBC that underwent resection, recurrence occurred in 66 (53%) [3]. Systemic chemotherapy is generally the sole option of treatment for recurrence; though metastatectomies have been performed in select cases for symptomatic control. In this report, we discuss a case of gallbladder cancer recurrence that presented as a circumferential intra-luminal colonic lesion, which posed a diagnostic challenge in differentiation from primary colorectal malignancy.
Case Report
This is a 69-year-old female retired nurse who presented to our institution’s colorectal surgery clinic for worsening abdominal pain associated with a newly discovered intraluminal colonic mass on colonoscopy. Her past medical history is significant for gallbladder cancer 3 years ago, diagnosed at an outside hospital after routine laparoscopic cholecystectomy for what was initially thought to be symptomatic cholelithiasis. The pathology of her gallbladder demonstrated findings consistent with adenocarcinoma, low-grade invasion into peri-muscular soft tissue, and was positive for lymphovascular and perineural invasion. No lymph nodes were obtained. For reasons unclear to the authors, she did not undergo repeat laparotomy for radical resection given her stage II disease (T2NxMx) and was instead followed clinically. Seven months later, she was found to have developed a 1.6cm enhancing lesion in the left hepatic lobe and was started on systemic chemotherapy with gemcitabine and capecitabine for 1.5 years without biopsy confirmation. She did well on this treatment with radiographic resolution of the liver lesion until 1 month prior to presentation, when she developed abdominal pain. A colonoscopy was performed that revealed a clearly intra-luminal, large circumferential friable mass in the sigmoid colon with luminal narrowing that did not allow passage of the scope. The lesion was biopsied and tattooed. Pathological evaluation of the lesion revealed invasive moderately differentiated, mucinous adenocarcinoma. At this point the origin of the tumor was unclear and the differential diagnosis included primary colorectal versus metastatic gallbladder. The patient was referred to our institution for management.
At presentation, she complained of diffuse abdominal pain without nausea or vomiting. She had at least one soft bowel movement daily, and reported 10 pounds of unintentional weight loss. Otherwise, her history was remarkable for diabetes, morbid obesity with BMI of 36, recurrent diverticulitis that resolved with medical management, and remote surgical history of hysterectomy. Her family history was remarkable for breast cancer (sister A age 40s) and colon cancer (sister B age 60s and sister C age 80s). She was a non-smoker and non-drinker. On physical exam, her abdomen was diffusely tender but soft without any peritoneal signs or any palpable mass. Her hemoglobin was slightly depressed at 11.1g/dL and carcinoembryonic antigen (CEA) level was elevated at 6.2ng/mL. As part of her work-up, she received a CT scan of the chest, abdomen, and pelvis that showed inflammatory stranding and fascial thickening surround the distal descending and sigmoid colon without any identifiable enlarged retroperitoneal or mesenteric lymph nodes. No hepatic lesions were seen. Notably there were numerous small pulmonary nodules bilaterally but were felt to be stable in comparison to prior imaging. Lastly, there was a 2.9cm right adrenal gland nodule. Due to incomplete colonoscopy, a barium enema was obtained that did not demonstrate any other colonic lesion other than severe narrowing at the descending colon (Figure 1).
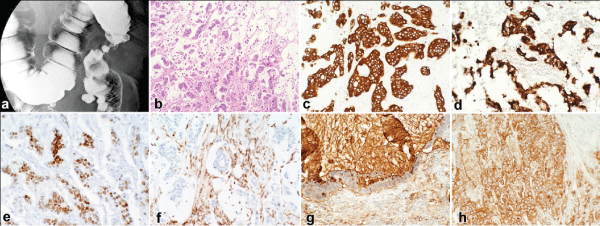
.
Figure 1: Radiologic and Histologic Findings of the Colonic Mass
a. Barium enema showing colonic constriction by the descending colon mass
b. H& E staining (10x) of invasive mucinous adenocarcinoma
c-h. Immunohistochemical staining of the colonic mass showing positive staining for CK7, CK20, CDX-2, SMAD4 (nuclear), CA19-9, and CEA, respectively (20x).
View Figure 1
This case was discussed at a multidisciplinary colorectal cancer conference, including a review of the relevant pathology at our institution. Consensus was not reached whether her colonic mass was a gallbladder cancer metastasis versus primary colon cancer. However, decision was made to proceed with surgical resection of the colonic mass.
The patient underwent laparoscopic left hemicolectomy approximately one month after presentation. Intraoperative findings include a 4.5cm firm, transmural mass encasing the descending colon, a 2.5cm hard umbilical nodule, and several sub-centimeter mesenteric/omental deposits. Microscopic examination of the specimen demonstrated morphology consistent with a mucinous adenocarcinoma. The surgical specimens were further investigated using immunohistochemical stains. The malignant cells were found to be positive for CK20, CK7, CDX-2, CEA, and CA19.9. Additionally, they showed loss of nuclear localization of SMAD-4. These immunohistochemical findings are shown in Figure 1. This profile correlated with her original gallbladder cancer specimen and thus secured a diagnosis of metastatic mucinous adenocarcinoma of the gallbladder (T2NxM1).
Postoperatively, the patient experienced superficial wound infection as well as readmission for small bowel obstruction that was conservatively managed. Eventually, she was started on systemic chemotherapy with gemcitabine. Unfortunately, at 3 months follow-up, she was noted to have marked clinical decline with an ECOG score of 3 and therefore referred to hospice care.
Discussion
Prognoses of patients with gallbladder cancer worsen dramatically beyond the early stage. After resection with curative intent, 5-year survival rates in patients with TNM Stage I-IV are 91%, 85%, 40%, and 19% respectively [4]. Dissemination of GBC has a propensity for distant spread. The initial site of recurrence is distant (72%) in most of the cases, and mostly in the peritoneum (31%) [3]. Rare metastases of GBC have been reported to the umbilicus, bone, breast, ovaries, cheek, and heart [5-9]. Aside from one case of GBC metastasis to the ileocecal valve, there has been no other report of GBC metastasis to the large intestine [10].
Because surgical intervention is unlikely to prolong survival, it is important to recognize progression of disease beyond the early stages. In this case, we were not able to do so initially due to several confounding factors. First the appearance of the colonic mass was clearly intra-luminal as shown in Figure 1. Metastatic lesions to the gastrointestinal tract usually present with obstructive-type symptoms due to external compression of an enlarging mass, where as this lesion appeared to be circumferentially constricting. Second, the patient had a strong family history of primary colon cancer. This, in addition to her well being after a non-biopsied liver lesion that resolved after chemotherapy and repeatedly negative surveillance imaging, argued for a higher likelihood for primary colorectal cancer. On the other hand, abdominal pain was her primary complaint, which is not typically associated with early primary colorectal cancer, where one would expect obstruction or bleeding. With knowledge of the patient’s oncologic history, our decision to operate was driven by the obstructive appearance of the lesion on colonoscopy. In retrospect, we feel that the patient likely developed an asymptomatic drop metastasis that became symptomatic as it eroded into colonic bowel wall. This is well supported by the fact that several other foci of metastases were not radiologically obvious but discovered upon entering her abdomen.
Faced with confounding information, pathological review of the resected specimen in this case was important in differentiating metastatic gallbladder cancer primary colon cancer. Morphologic evaluation alone was insufficient for diagnosis, because mucinous adenocarcinoma exists as a subtype in both gallbladder (5-10%) and colorectal cancer (5-15%), associated with a poorer prognosis [11,12]. Instead, immunohistochemical investigation proved to be critical, including staining for CA19-9, CEA, CDX-2, CK7, and CK20. CA19-9 and CEA are mucins whose expression is common to both GBC and CRC. CEA expression occurs in 75% of T2-4 GBC is correlated with growth and metastasis of GBC [13]. CDX-2 is an intestinal transcription factor and a marker of intestinal differentiation; it is rare in pancreatobiliary adeno carcinomas compared with colonic adeno carcinomas, where is very common [14]. CK7 is a subtype of high-molecular-weight cytokeratins, is expressed in pancreatobiliary ductal epithelium and its neoplasms [15]. CK20 is a subtype of low-molecular-weight cytokeratins, and is expressed in intestinal-type epithelium and its neoplasms [16,17]. From existing literature we collected known positive frequencies of these stains in gallbladder versus colorectal cancer, as shown in Table 1. From this information we were able to calculate the probability of the colonic mass representing a new colorectal primary tumor expressing all of these markers at 0.75-12.4%. In addition, we ran the same immune histochemical studies on all samples dating back to her initial gallbladder specimen. Although this particular combination of markers was rare for GBC (accounting for 1-7% of all GBC), the results demonstrated concordance of the immune phenotype in all specimens. Given the low probability that a new primary colorectal mass would have this particular expression pattern, coupled with the high probability that a metastatic carcinoma would maintain its immune phenotype strongly suggests that the colorectal mass is a metastasis from the primary gallbladder carcinoma.
![]()
Table 1: Relative frequencies of marker positivity in gallbladder and colorectal cancer
View Table 1
In review of this case, a few elements of this patient’s care could have been optimized. First, her initial surgical management of stage II gallbladder cancer without radical resection was suboptimal. There is ample evidence that radical surgery with inclusion of partial hepatectomyis associated with improved survival in advanced staged patients, and could potentially be curative in her stage II disease. Second, immune histochemical review of the colonoscopy specimen was not initially performed. If this had occurred, perhaps the diagnosis of stage IV GBC could have been secured earlier and led to earlier initiation of systemic therapy for control of abdominal pain. This is especially important because while the patient was not symptomatically obstructed, she underwent colon resection and developed a wound infection and required readmission for small bowel obstruction postoperatively. Ultimately, her surgery likely delayed initiation of chemotherapy and did not affect her overall survival.
In conclusion, we used molecular diagnostic tools and our review of the literature to evaluate a diagnostically challenging and extremely rare case of metastatic gallbladder cancer masquerading as primary colorectal tumor. In such cases, communication between the pathologist and surgeon proves absolutely crucial in the effective diagnosis and management of rare presentations of surgical diseases.
Disclaimers
Statement that patient consent was obtained: No patient identifiers were used in this report
References
-
Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, et al. (2001) Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 51: 349-364.
-
Reid KM, Ramos-De la Medina A, Donohue JH (2007) Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg 11: 671-681.
-
Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, et al. (2003) Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 98: 1689-1700.
-
Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, et al. (1996) Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery 120: 816-821.
-
Carlomagno C, Insabato L, Bifulco G, De Placido S, Lauria R (2010) Ovarian metastasis following gallbladder carcinoma: a case report. Eur J Gynaecol Oncol 31: 219-221.
-
Gupta M, Rastogi N, Lal P (2003) Carcinoma of the gallbladder with unusual umbilical metastasis. Lancet Oncol 4: 319-320.
-
Inoue T, Shiraki K, Fuke H, Yamanaka Y, Miyashita K, et al. (2005) Cardiac metastases of gallbladder carcinoma. World J Gastroenterol 11: 2048-2049.
-
Singh S, Bhojwani R, Singh S, Bhatnagar A, Saran RK, et al. (2007) Skeletal metastasis in gall bladder cancer. HPB (Oxford) 9: 71-72.
-
Singh S, Gupta P, Khanna R, Khanna AK (2010) Simultaneous breast and ovarian metastasis from gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 9: 553-554.
-
Virgilio E, Giaccaglia V1, Balducci G1 (2014) Re: metastasis of gallbladder adenocarcinoma to Bauhin's valve: an extremely rare cause of intestinal obstruction. Korean J Radiol 15: 655-656.
-
Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C et al. (2001) Epidemiology and Molecular Pathology of Gallbladder Cancer. CA: A Cancer Journal for Clinicians 51:349-364.
-
Dursun N, Escalona OT, Roa JC, Basturk O, Bagci P, et al. (2012) Mucinous carcinomas of the gallbladder: clinicopathologic analysis of 15 cases identified in 606 carcinomas. Arch Pathol Lab Med 136: 1347-1358.
-
Fante R, Benatti P, di Gregorio C, De Pietri S, Pedroni M, et al. (1997) Colorectal carcinoma in different age groups: a population-based investigation. Am J Gastroenterol 92: 1505-1509.
-
Dowaki S, Kijima H, Kashiwagi H, Ohtani Y, Tobita K, et al. (2000) CEA immunohistochemical localization is correlated with growth and metastasis of human gallbladder carcinoma. Int J Oncol 16: 49-53.
-
Li MK, Folpe AL (2004) CDX-2, a new marker for adenocarcinoma of gastrointestinal origin. Adv Anat Pathol 11: 101-105.
-
Lee MJ, Lee HS, Kim WH, Choi Y, Yang M (2003) Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol 16: 403-410.
-
Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. (2002) Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol: 21: 391-400.
-
Agrawal V, Goel A, Krishnani N, Pandey R, Agrawal S, et al. (2010) p53, carcinoembryonic antigen and carbohydrate antigen 19.9 expression in gall bladder cancer, precursor epithelial lesions and xanthogranulomatous cholecystitis. Journal of postgraduate medicine 56 : 262-266.
-
Brown RW, Campagna LB, Dunn JK, Cagle PT (1997) Immunohistochemical identification of tumor markers in metastatic adenocarcinoma. A diagnostic adjunct in the determination of primary site. American journal of clinical pathology 107: 12-19
-
Maeda T, Kajiyama K, Adachi E, Takenaka K, Sugimachi K, et al. (1996) The expression of cytokeratins 7, 19, and 20 in primary and metastatic carcinomas of the liver. Mod Pathol 9: 901-909.
-
Dowaki S, Kijima H, Kashiwagi H, Ohtani Y, Tobita K, et al. (2000) CEA immunohistochemical localization is correlated with growth and metastasis of human gallbladder carcinoma. Int J Oncol 16: 49-53.
-
Owens CL, Epstein JI, Netto GJ (2007) Distinguishing prostatic from colorectal adenocarcinoma on biopsy samples: the role of morphology and immunohistochemistry. Arch Pathol Lab Med 131: 599-603.
-
Park SY, Kim BH, Kim JH, Lee S, Kang GH (2007) Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med 131: 1561-1567.
-
Barbareschi M, Murer B, Colby TV, Chilosi M, Macri E, et al. (2003) CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol 27: 141-149.
-
Hughes NR, Bhathal PS (2013) Adenocarcinoma of gallbladder: an immunohistochemical profile and comparison with cholangiocarcinoma. J Clin Pathol 66: 212-217.
-
Lee MJ, Lee HS, Kim WH, Choi Y, Yang M (2003) Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol 16: 403-410.
-
Lau SK, Prakash S, Geller SA, Alsabeh R (2002) Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Human pathology 33 :1175-1181.
-
Lewis MR, Deavers MT, Silva EG, Malpica A (2006) Ovarian involvement by metastatic colorectal adenocarcinoma: still a diagnostic challenge. Am J Surg Pathol 30: 177-184.





