Clinical Medical Reviews and Case Reports
Anti Citrullinated Protein/Peptide Antibody Assay, Rheumathoid Factor or Both as Shifted Test in Diagnostic and Prognostic Evaluation in Patients with Rheumathoid Arthritis
Dejan Spasovski1* , Tatjana Sotirova2, Svetlana Krstevska-Balkanov2, Maja-Slanin-ka-Micevska3, Trajan Balkanov3, Sonja Alabakovska4 and Sonja Genadieva-Stavric2
1Department of Rheumatology, University Clinical Centre, Skopje, Republic of Macedonia
2University Clinic of Heamatology, Cyril and Methodius University, Skopje, Republic of Macedonia
3Department of Preclinic Farmacology, University Clinical Centre, Skopje, Republic of Macedonia
4Department of Biochemistry, University Clinical Centre, Skopje, Republic of Macedonia
*Corresponding author: Dejan Spasovski, Deparment of Rheumatology, University Clinical Centre, Skopje, Republic of Macedonia, E-mail: drspasovski@yahoo.co.uk
Clin Med Rev Case Rep, CMRCR-1-004, (Volume 1, Issue 1), Original Article; ISSN: 2378-3656
Received: August 22, 2014 | Accepted: October 22, 2014 | Published: October 27, 2014
Citation: Spasovski D, Sotirova T, Balkanov SK, Micevska MS, Balkanov T, et al. (2014) Anti Citrullinated Protein/Peptide Antibody Assay, Rheumathoid Factor or Both as Shifted Test in Diagnostic and Prognostic Evaluation in Patients with Rheumathoid Arthritis. Clin Med Rev Case Rep 1:004. 10.23937/2378-3656/1410004
Copyright: © 2014 Spasovski D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: The aim of this study was to compare the diagnostic values of laboratory variables, to present quantitative evaluations of the anti citrullinated protein / peptide antibody (ACPA), or anti CCP ( anti-cyclic citrullinated peptide, anti-CCP 2) antibodies in second generation antibody assay diagnostic test with reference to sensitivity and specificity, the predictive value of the positive and negative test and precision of the test for ACPA antibodies, rheumatoid factor, C-reactive protein and DAS 28 index, in the early diagnosis of untreated rheumatoid arthritis.
Materials and methods: 70 participants (35 patients with rheumatoid arthritis not treated, 35 individuals as healthy controls) took part in the study. Their serum was examined using ELISA technology of DIA-STATTM Anti-CCP (Axis-Shield Diagnostics). Rheumatoid factor was examined with the test for agglutination (Latex RF test).
Results: We found the presence of ACPA antibodies (sensitivity of the test 65.71%) in 23 of the 35 examined patients with rheumatoid arthritis while rheumatoid factor appeared in 17 patients (sensitivity of the test 48.57%). Twelve patients were ACPA and rheumatoid factor positive, 11 were ACPA positive, but rheumatoid factor negative. Five patients were ACPA negative and rheumatoid factor positive. In 17 rheumatoid factor positive patients, ACPA antibodies were positive in 12 patients. Of 18 rheumatoid factor negative patients, 11 were ACPA positive. In the healthy control group, 1 patient was anti-CCP 2 positive, while 2 patients were rheumatoid factor positive.
Conclusion: ACPA antibodies have higher sensitivity and specificity than rheumatoid factor in rheumatoid arthritis.
Keywords
Rheumatoid arthritis, Rheumatoid factor, ACPA antibody
Introduction
Rheumatoid Arthritis (RA) is an autoimmune disease, multi-functional in origin, characterised by the inflammation of the membrane lining joints. The disease spreads from small to large joints, with the greatest damage in the early phase [1]. The diagnostics of RA is based on clinical, radiological and immunological features. The most frequent serological test is the measurement of Rheumatoid Factor (RF). American College of Rheumatology for the classification of RA comprise RF as one of its criteria. The most common class is IgM and it is found in 60-80% of RA patients. RF is not specific for RA, as it is often present in healthy individuals and patients with other autoimmune diseases and chronic infections [2]. 30% of patients with SLE are RF positive (with no evidence of RA) [4]. Despite its low specificity, a positive RF is considered an important predictor of outcome in RA. Antibodies to anti-perinuclear factor (APF) and keratin (AKA) are considered as highly specific for RA. Antibodies to APF and AKA were detected by indirect immune fluorescence using buccal epithelium of rat oesophagus [4]. The lack of availability of suitable buccal cell donors has limited the use of APF as a routine laboratory test. The antigen of both these antibodies has been identified as epidermal filaggrin, an intermediate filament-associated protein involved in the cornification of the epidermis [5,6]. Profilagrin, present in the keratohyaline granules of human buccal mucosa cells, is proteolytically cleaved into filaggrin subunits during cell differentiation. The protein is dephosphorylated and some arginine residues are converted to citrulline by the enzyme peptidylarginine deaminase (PAD) [7]. Auto antibodies reactive with linear synthetic peptides containing the unusual amino citrulline were present in 76% of RA sera with specificity for RA of 96% [8]. The antibodies in patients with RA that recognized the citrulline containing epitopes were predominantly of the IgG class and of relatively high affinity [8]. In a subsequent paper [9] it is reported that the ELISA test based on Cyclic Citrullinated Peptide (CCP) showed superior performance characteristics to one based on the linear version in the detection of antibodies to RA. In principle, most citrullinated protein/peptides are recognized by auto antibodies in RA sera, although with different sensitivities and specificities [10]. These findings suggest an important role of citrullinated antigens in the diagnosis of RA. Sensitivity of the anti-CCP 2 test varies between 64% and 74% in different populations, but the specificity varies between 90% and 99% [11-16].
Material and Methods
The diagnosis of the RA was established on the basis of the revised diagnostic criteria for classification of rheumatoid arthritis, suggested in 1987 by the American Association for Rheumatism (ARA) [17]. To be diagnosed as patient with RA one must fulfil at least four out of seven criteria. Criteria from one to four should be present for at least six weeks.
70 participants were included in the study: 35 patients with newly diagnosed RA, not treated (28 females, 7 males) and 35 individuals as healthy control group (18 females, 17males), aged 18-65 years. The average age was 56.68 years (± 6.79) (40-65 years) in the RA group and 46.2 years (± 12.49) (29-65 years) in the healthy control group. The average duration of the disease in months was 43.97 (± 45.23), in the interval of 1-168 months. All the participants included in the study denied medical history of renal disease.
Patients with disease or condition which could directly or indirectly influence any change in the results were excluded from the study:
1. Patients with SLE, Sjogren syndrome, mixed conjunction tissue disease, vasculitis, autoimmune disease, age < 18 years.
2. Patients treated with antibiotics and salycilate in periods under six months from the beginning of the study.
3. Patients who together with these medicines took medicines from basic line.
4. Patients with previous medical history of disease of the spleen, thyroid gland, liver damage, renal, hematologic, arterial hypertension, uric arthritis, uric infections, cardiovascular, neurologic and lung impairment, AIDS,.
5. Patients with diabetes mellitus, acute infections, malignant neoplasm, febrile conditions.
6. Patients treated with antihypertensive, diabetic and cardiac therapy.
7. Hypersensitive to some of the medicines or their components.
8. Patients with previous history of transfusion of blood and overweight.
9. Patients whose results showed that in 0 spot there was a glycemia, or increased level of degraded products as creatinine in serum and urine, urea in serum and disorder of the hematologic and enzymatic status.
All patients took part in this study voluntarily, so the ethical criteria were fulfilled.
Clinical evaluation of disease activity
The clinical evaluation was performed by the subspecialist in this field did. The disease activity was evaluated using DAS 28 index (Disease Activity Score, DAS 28) [18-21]. The index is a mathematical formula that allows to get a uniquely composed quantitative score, which comprise palpation - painful sensitive joints (max number 28), swollen joints (max number 28), Westergren's Erythroid Sedimentation Rate (ESR), and patient's global assessment of disease activity (0-100 mm Visual Analogous Scale VAS) and the morning rigidity (minutes). DAS 28 index is ranked from 0 to 10 and a score under 3.2 ranks the disease as low active.
Laboratory assessment
Several laboratory variables have to be measured for a clinical assessment of the basic disease: Complete Blood Count (CBC) and differential, reactors of acute phase - RF, CRP, anti-CCP 2, Alkaline Phosphatase (AP), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Creatinine Kinase (CK), Lactate Dehydrogenase (LDH), serum urea and creatinine.
The DIA-STATTM Anti-CCP (Axis-Shield Diagnostics) test is a semi quantitative/qualitative Enzyme- Linked Immuno sorbent Assay (ELISA) for the detection of the IgG class of auto antibodies specific to synthetic Cyclic Citrullinated Peptide (CCP) containing modified arginine residues. The test provides an additional tool in the diagnosis of patients with RA.
The absorbance value (optical density ratio) for the positive and negative control and for each sample was calculated. The recommended values for the test are:
| Absorbance ratio | Result interpretation |
| < 0.95 | Negative |
| >0.95 to < 1.0 | Borderline-recommended repeat testing |
| >1.0 | Positive |
Reference values are: under 1,26 U/ml ACPA in serum.
The test of agglutination (Latex CRP test) (BioSystems S.A. Reagents&Instruments Costa Brava 30, Barcelona, Spain) was used for determination of CRP [22-26]. Reference values are: under 6 mg/L CRP in serum.
RF was detected with the test of agglutination (Latex RF test) (BioSystems S.A. Reagens& Instruments Costa Brava 30, Barcelona, Spain) [22,26-30]. Reference values are: under 8 mg/L RF in serum.
For determination of ESR we used the method after Westergren, and normal values are: 7-8 mm for males, 11- 16 mm for females.
Statistical analysis
The Student's t-test was used for testing the importance of the difference between two arithmetic means, with respect to proportion, which compares the middle values of certain numerical parameters between two groups. Wilcoxon-matched test was used for independent samples. Sensitivity and predictivity were defined for positive and negative test of examined values. P value between 0.05 and 0.1 was taken as statistically significant. Data processing was done with the statistical package - Statistica 7.0
Results
Out of 35 patients with RA, RF was present in 17 patients (48.57%), while 23 patients (65.71%) showed presence of ACPA antibody, 12 patients were ACPA and RF positive (34.28%), 11 patients (31.42%) were ACPA positive and RF negative, while 5 patients (14.28%) were ACPA negative and RF positive. Of 18 RF negative patients, 11 patients (61.11%) were ACPA positive. Out of the total of 12 ACPA negative RA patients, 5 patients (41.66%) were RF positive. Of 35 examined patients with RA, sensitivity to ACPA was 65.71%, while RF sensitivity was 48.57%. Of 17 RF positive RA patients, ACPA antibody was present in 12 patients and its sensitivity was 70.58%. Out of 18 RF negative RA, ACPA was present in 11 patients and its sensitivity was 61.11%. In the healthy control group 2 participants (5.71 %) were RF positive, while 1 (2.85%) was ACPA positive (Table 1).
![]()
table 1:Anti CCP 2 antibody and RF in RA and healthy control group.
View Table 1
Diagnostic performance of ACPA antibody in patients with RA
For ACPA antibody and RF in RA, sensitivity, specificity, predictive value of the positive and negative tests as well as their precision are shown in table 2. ACPA antibodies showed better diagnostic performance than RF (sensitivity 65.71% vs. 48.57%, specificity 97.14% vs. 94.28%) in the detection of RA.
![]()
Table 2:Diagnostic performance of ACPA antibody and RF in rheumatoid arthritis.
View Table 2
Corelaion between ACPA antibody and DAS 28 index of activity of disease
Out of 35 patients with RA, DAS 28 > 3.2 was replaced in 28 patients (80%). In 17 seropositive RF patients, replacement of DAS 28 > 3.2 was found in 15 patients (88.23%). Among these 15 patients with DAS 28 > 3.2, 10 were ACPA positive (66.66%), and their M ± SD (2.23 ± 0.61) was extended (1.28-3.0). In 18 seronegative RF patients, replacement of DAS 28 > 3.2 was found in 13 patients (72.22%). Among these 13 patients with DAS 28 > 3.2, 9 were ACPA positive (69.23%) and their M ± SD (1.92 ± 0.45) was extended (1.3-2.6). Seropositive RF patients have higher titer of ACPA antibody than RF seronegative (Table I), (1.87± 0.77 (0.92-3.0) vs. 1.56 ± 0.59 (0.93-2.6)), and a higher DAS 28 > 3.2 index (5.04 ±1.33 (2.47-6.83) vs. 4.56 ± 1.76 (1.85- 7.03)). Between these two groups of ACPA antibody there was no statistical relation (p = 0.266). Although the same representation of ACPA positive patients with DAS 28 > 3.2 was found in seropositive and seronegative patients (10 vs. 9 patients; 66.66% vs. 69.23%), the titer of ACPA was higher in 10 RF seropositive patients with DAS 28 > 3.2, compared with RF seronegative patients with DAS 28 > 3.2 (2.23 ± 0.61 vs. 1.92 ± 0.45). Between these two groups there was no statistical correlation (p = 0.374260) (Figure 1). The condition was almost equal for DAS 28 index in 9 RF seronegative, ACPA positive patients (5.69 ± 1.37) extent 3.31-7.03 compared with 10 RF seropositive ACPA positive patients (5.63 ±1.01) extent 4.17-6.83. There was no statistical correlation between DAS 28 index in RF seropositive and seronegative patients (p = 0.379375) and between two groups of DAS 28 > 3.2, ACPA positive patients, but RF seropositive and seronegative patients (p = 0.905696) (Figure 2).
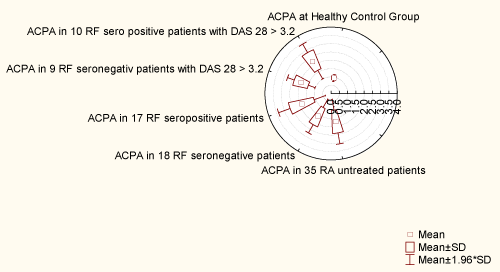
Figure 1: Distribution of ACPA antibody
View Figure 1
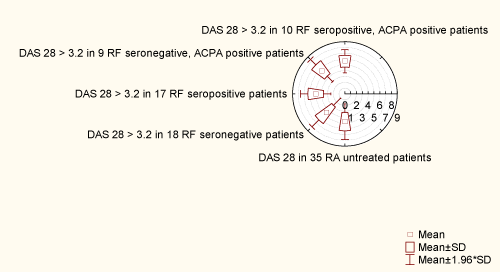
Figure 2: Distribution of das 28 index of activity of disease
View Figure 2
A statistical correlation was found using Wilcoxon - matched test between ACPA in RA and healthy control group for p< 0.05 (p = 0.000002). A statistical correlation was found using Wilcoxonmatched test between: ACPA in RA and DAS 28, RF and CRP, SER, morning rigidity in the same group for p< 0.05: (anti-CCP 2 vs. DAS 28 p = 0.000000; ACPA vs. RF (p = 0.018345); ACPA vs. CRP p = 0.040620; anti-CCP 2 vs. morning rigidity (p = 0.000032); ACPA vs. ESR (p = 0.000000).
Discussion
It is reported that sensitivity of first generation anti-CCP antibody is approximately 68% (45-80%) and specificity is 98% (96-100%). (9) The report for the sensitivity of second [2] generation anti-CCP 2 antibody is approximately 64-74%, and the specificity is 90-99% [11-16,31,32]. The advantages of the use of anti-CCP 2 test can be seen in the early phase of arthritis [33]. Our conclusions for sensitivity of 65.71% and specificity of 97.14% are similar to these studies. High specificity (61.11%) was found in RF negative RA patients. Mean sensitivity and high specificity allow ACPA antibody to be included as a classification criterion in RA. Although DAS 28 index, which is not only a laboratory variable, but also a clinical index for evaluation of disease, has higher sensitivity (80%) and specificity (100%), ACPA antibody as an isolated laboratory variable, dominated with its performances in the early diagnosis of undifferentiated RA. However, we have to pay attention to the fact that the results obtained in this study are lower and retreat from values given by the producer DIA-STATTM Anti-CCP (Axis-Shield Diagnostics) (sensitivity for anti-CCP 2 79%, specificity 100 %). Data obtained for ACPA antibody were higher than those from tests by other examiners [12,31,34].
It is known that the keratohyalin bodies present in human buccal mucosa cells contain filaggrin, a protein that is recognized by APF and AKAs specific antibodies present in RA patients. These antibodies are detectable by indirect immune fluorescence techniques, but they have never become part of the diagnostic repertoire of clinical laboratories because of difficulties in the availability and storage of the antigen substrates, as well as objective difficulties in interpreting the fluoroscopic patterns.
The recent development of synthetic peptides containing citrulline [8], an amino acid present in the filaggrin molecule and produced after its deimination, has enabled the development of an ELISA test. From preliminary data obtained during experimental trials, this test appears to have the same high specificity as APF and AKAs and is able to eliminate the standardization problems related to immunofluorescence procedures. In this study, we evaluated the diagnostic accuracy of this new ELISA test, which is now commercially available.
The sensitivity of first generation anti-CCP 2 antibodies is reported to be approximately 68% (45-80%) and specificity is 98% (96-100%) [9]. The report for sensitivity of the second [2] generation anti-CCP 2 antibody is approximately 64-74%, with the specificity of 90-99% [11-16,32]. The advantages of the use of anti-CCP 2 test might be seen as a possibility of an early differentiation of arthritis.(33) Our findings for specificity of 65.71% and specificity of 97.14% are in line with the frames of others studies. In addition, a high specificity is useful in RF negative RA patients, where it is 61.11%. Mean sensitivity and high specificity allow anti-CCP 2 antibody to be included as a classification criterion in RA. Although the DAS 28 index, which is not only a laboratory variable but a clinical index for the estimate of the disease, has higher sensitivity (80%) and specificity (100%), anti-CCP 2 antibody, as an isolated laboratory variable, dominates with its performance in the early diagnosis of undifferentiated RA. However, we have to pay attention to the fact that the results achieved in this study are bellow the values given by the producer DIA-STATTM Anti-CCP (Axis-Shield Diagnostics) (sensitivity for anti-CCP 2 79%, specificity 100 %). Data given for ACPA antibody are higher than those from previous tests by other examiners [12,31,34].
The efficacy of anti-cyclic citrullinated peptide (anti-CCP) antibody detection in the early diagnosis of RA is shown by Fernandez-Suarez A, et al [31], as are compared three commercially available Enzyme-Linked Immunoabsorbent Assay (ELISA) kits used for detection of such antibodies. The presence of anti-CCP antibodies was analysed in the sera of 78 patients, newly diagnosed. A group of 50 healthy controls was also included in the study. None of them had previously been treated. After follow-up of 1-year, diagnosis of RA was confirmed in 53 patients. The ELISA kits used in the study were IMMUNOSCAN RA (Euro-Diagnostica AB). QUANTA Lite CCP IgG ELISA (INOVA Diagnostic) and DIA-STAT Anti-CCP (Axis-Shield Diagnostics). The sensitivity was 52,8% 58,5% and 52,8%, respectively, and specificity 100% for all three kits. Anti-CCP antibodies detected the presence of RA in 26% RF negative patients. The sum of anti-CCP antibodies of the presence of RF gave a sensitivity of up to 67%, with specificity ranging between 94 and 97%. It was shown that anti-CCP antibodies had high specificity for the diagnosis of RA. There was no difference in terms of diagnostic accuracy among the three analysed ELISAs.
The presence of anti-CCP antibodies in RA suspected patients was investigate by Us D et al. [34] They evaluated the combination of these autoantibodies with some other serologic markers such as IgM-rheumatoid factor (IgM-RF), CRP and Antinuclear Antibodies (ANAs). The concentrations of RF and CRP were determined by quantitative immune nephelometry; titers of ANAs by indirect immune fluorescence and the presence of anti-CCP by a commercial semi quantitative micro ELISA method. 88 patients with clinically suspected RA were analysed, as well as 42 sex- and age-matched healthy blood donors. High levels of IgM-RF and CRP were found in 48 (54.5%) and 49 (55.7%) patients, respectively, while 47 (53.4%) and 25 (28.4%) patients were found positive for ANAs and anti-CCP, respectively. Of 48 RF positive patients, 25 were also positive for anti-CCP and distribution rates of the markers in 25 anti-CCP positive patients were as follows: 100% for RF, 84% for CRP and 52% for ANA. The sensitivity of anti-CCP ELISA was 52.1% and specificity was 100%, when evaluated according to RF positivity as a main serologic marker of RA.
In order to explain the low sensitivity, it has to be taken in consideration that anti-CCP antibodies are a heterogeneous group of antibodies directed against different epitopes on the citrulline molecule, that each patient's serum contains different subsets of antibodies, and that the synthetic peptide used in this assay represents a relatively small set of antigenic determinants that do not entirely encompasses the antigenic determinants present on the yet unknown antigenic molecule in the joint [35].
ACPA and RF in RA patients were also evaluated in terms of duration of disease. In patients with early arthritis the correlation with anti-CCP was highly significant, indicating that this assay may be useful even in the early phase of disease. It is important because an early diagnosis of RA could modify in a great deal treatment decision, suggesting use of more aggressive drugs that can delay progression of joint damage and thus substantially change the natural history of disease.
We can conclude that ACPA antibody assay is a very valuable test for diagnosis of RA. This ELISA test surpasses many of the problems of the APF and AKA tests, such as quantification of the results and standardization of the assay. Its low sensitivity does not allow its use as a screening test, but its high specificity, especially in the presence of high concentrations, allows it to become one of the most useful serologic tests for diagnosis of RA. When associated with RF determination, its specificity rises up to 100%, make it helpful in the differential diagnosis of RA and other rheumatic diseases. This test may be very influential in treatment decision strategy in patients with recent onset of arthritis.
Anti-CCP 2 antibodies have higher sensitivity and specificity than RF in RA. Anti-CCP 2 test is used in everyday clinical practice for the diagnosis of early undifferentiated RA.
References
-
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344: 23-27.
-
Smolen JS, Butcher B, Fritzler MJ, Gordon T, Hardin J, et al. (1997) Reference sera for antinuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and western blotting. Arthritis Rheum 40: 413-418.
-
Barland P, Lipstein E (1996) Selection and use of laboratory tests in the rheumatic diseases. Am J Med 100: 16S-23S.
-
Nakamura RM (2000) Progress in the use of biochemical and biological markers for evaluation of rheumatoid arthritis. J Clin Lab Anal 14: 305-313.
-
Simon M, Girbal E, Sebbag M, Gomes-Daudrix V, Vincent C, et al. (1993) The cytokeratin filament-aggregating protein filaggrin is the target of the so-called "antikeratin antibodies," autoantibodies specific for rheumatoid arthritis. J Clin Invest 92: 1387-1393.
-
Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, et al. (1995) The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest 95: 2672-2679.
-
Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, et al. (1999) The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol 162: 585-594.
-
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101: 273-281.
-
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, et al. (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43: 155-163.
-
van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ (2002) Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res 4: 87-93.
-
Lee DM, Schur PH (2003) Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 62: 870-874.
-
Suzuki K, Sawada T, Murakami A, Matsui T, Tohma S, et al. (2003) High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol 32: 197-204.
-
Dubucquoi S, Solau-Gervais E, Lefranc D, Marguerie L, Sibilia J, et al. (2004) Evaluation of anti-citrullinated filaggrin antibodies as hallmarks for the diagnosis of rheumatic diseases. Ann Rheum Dis 63: 415-419.
-
Kastbom A, Strandberg G, Lindroos A, Skogh T (2004) Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 63: 1085-1089.
-
Vallbracht I, Rieber J, Oppermann M, Forger F, Siebert U, et al. (2004) Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 63: 1079-1084.
-
Zendman AJ, van Venrooij WJ, Pruijn GJ (2006) Use and significance of anti-CCP autoantibodies in rheumatoid arthritis. Rheumatology (Oxford) 45: 20-25.
-
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. (1998) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315-324
-
van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, et al. (1996) Development and validation of the european league against rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary american college of rheumatology and the world health organization/international league against rheumatism criteria. Arthritis Rheum 39: 34-40.
-
Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, et al. (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38: 44-48.
-
Balsa A, Carmona L, Gonzalez-Alvaro I, Belmonte MA, Tena X, et al. (2004) Value of Disease Activity Score 28 (DAS28) and DAS28-3 compared to American College of Rheumatology-defined remission in rheumatoid arthritis. J Rheumatol 31: 40-46.
-
Prevoo ML, van Gestel AM, van T Hof MA, van Rijswijk MH, van de Putte LB, et al. (1996) Remission in a prospective study of patients with rheumatoid arthritis. American rheumatism association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 35: 1101-1105.
-
Friedman RB, Young DS, Beatty ES (1978) Automated monitoring of drug-test interactions. Clin Pharmacol Ther 24: 16-21.
-
Turgeon ML, Mosby JA (1996) Immunology and serology in laboratory medicine,2nd edition 2: 485-489.
-
SINGER JM, PLOTZ CM, PADER E, ELSTER SK (1957) The latex-fixation test. III. Agglutination test for C-reactive protein and comparison with the capillary precipitin method. Am J Clin Pathol 28: 611-617.
-
Hokama Y, Nakamura RM (1987) C-Reactive protein: current status and future perspectives. J Clin Anal 1: 15-27.
-
Young DS, Thomas DW, Friedman RB, Pestaner LC (1972) Effects of drugs on clinical laboratory tests. Clin Chem 18: 1041-1303.
-
PLOTZ CM, SINGER JM (1956) The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am J Med 21: 888-892.
-
Shmerling RH, Delbanco TL (1991) The rheumatoid factor: an analysis of clinical utility. Am J Med 91: 528-534.
-
Sager D, Wernick RM, Davey MP (1992) Assays for rheumathoid factor:a review of their utility and limitation in clinical practise. Lab Med 23: 15-18.
-
Burtis CA, Ashwood ER (1999) Quality management. Tietz textbook of clinical chemistry, 3rd edition1095-1124. WB Saunders Co.
-
Fernandez-Suarez A, Reneses S, Wichmann I, Criado R, Nunez A (2005) Efficacy of three ELISA measurements of anti-cyclic citrullinated peptide antibodies in the early diagnosis of rheumatoid arthritis. Clin Chem Lab Med 43: 1234-1239.
-
De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, et al. (2004) Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate and extra-articular manifestations. Ann Rheum Dis 63: 1587-1593.
-
Van Gaalen FA, Linn-Rasker SP, Van Venrooij WJ, de Jong BA, Breedveld FC, et al. (2004) Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 50: 709-715.
-
Us D, Gulmez D, Hascelik G (2003) [Cyclic citrullinated peptide antibodies (anti-CCP) together with some other parameters used for serologic diagnosis of rheumatoid arthritis]. Mikrobiyol Bul 37: 163-170.
-
van Jaarsveld CH, ter Borg EJ, Jacobs JW, Schellekens GA, Gmelig-Meyling FH, et al. (1999) The prognostic value of the antiperinuclear factor, anti-citrullinated peptide antibodies and rheumatoid factor in early rheumatoid arthritis. Clin Exp Rheumatol 17: 689-697.





