Clinical Medical Reviews and Case Reports
Anesthetic Management of Traumatic Brain Injury
Hashim Qureshi1, Hussain Mithaiwala1, Jacob Ezell1 and Marco Maurtua2*
1House Staff, Anesthesiology Institute, Cleveland Clinic Foundation, USA
2Staff Anesthesiologist, Anesthesiology Institute, Cleveland Clinic Foundation, USA
*Corresponding author:
Marco Maurtua, M.D., Staff Anesthesiologist, Anesthesiology Institute, The Cleveland Clinic Foundation, 9500 Euclid Avenue E-30, Cleveland, Ohio 44195, USA, Tel: +216-445-1151, E-mail: maurtum@ccf.org
Clin Med Rev Case Rep, CMRCR-4-159, (Volume 4, Issue 3), Original Review; ISSN: 2378-3656
Received: November 23, 2016 | Accepted: March 25, 2017 | Published: March 27, 2017
Citation: Qureshi H, Mithaiwala H, Ezell J, Maurtua M (2017) Anesthetic Management of Traumatic Brain Injury. Clin Med Rev Case Rep 4:159. 10.23937/2378-3656/1410159
Copyright: © 2017 Qureshi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Worldwide, Traumatic Brain Injury (TBI) is a leading cause of morbidity and mortality. It is the third most common cause of injury related death with direct and indirect costs totaling an estimated 60 billion dollars annually in the United States. Management of a TBI patient is guided by recommendations made by the Brain Trauma Foundation (BTF). Despite advances made in care, there is still a high mortality rate depending on severity of injury. Knowing when to obtain imaging for TBI patients can prevent excessive strain on hospital resources. There continues to be a debate on how to secure an airway in patients suspected to have a cervical spine injury. The purpose of this review article is to highlight initial management of a TBI patient, securing an airway, and recommendations for intraoperative management.
Introduction
Traumatic Brain Injury (TBI) is a leading cause of morbidity and mortality worldwide [1]. In the United States alone, around 1.3 million Emergency Department visits occur annually for TBI. Nearly 275,000 of these visits result in hospitalization while another 52,000 cases result in death [1]. The vast majority of fatal TBI cases are attributed primarily to firearms (39%) and motor vehicle accidents (34%) and the majority of non-fatal cases of TBI are attributed to falls. Therefore it is not surprising that the most commonly affected age groups are children less than 14 and adults over 65, where falls are common [1,2]. As the third most common cause of injury related death, an estimated 60 billion dollars are spent managing TBI patients in the United States per year [1].
Despite new medical advances in monitoring and treatment strategies, outcomes after TBI remain poor. According to the Brain Trauma Foundation (BTF), the use of evidence based protocols has reduced TBI mortality from 50% to 25% over the last three decades [3,4]. The United States Department of Defense developed a new way to classify patients who suffered from TBI, into mild, moderate, and severe based on Glasgow Coma Score (GCS), duration of post-traumatic amnesia, and presence or absence of loss of consciousness, to be able to more accurately predict outcome [5]. The purpose of this review article is to highlight major areas of intervention during the perioperative period of TBI patients and to prevent complications arising from the primary and or secondary mechanisms of injury.
Pathophysiology of TBI
The pathophysiology of TBI is divided into primary and secondary injuries. The primary injury is the initial injury due to physical or mechanical forces on the brain parenchyma and skull [6]. This primary injury leads to an inflammatory cascade including cerebral edema, axonal injury, and decreased cerebral perfusion pressure [6]. Secondary injuries include, electrolyte abnormalities, hypoxemia, glycemic imbalance, hypotension, increased Intracranial Pressure (ICP), and hyper or hypocarbia. Secondary injuries are generally a consequence of the primary injury [6]. Patient outcomes correlate with the severity of the primary injury. Thus, rapid and efficient TBI severity stratification will allow appropriate intervention during the perioperative period to attenuate the effects of primary injuries and prevent secondary injuries [7].
Evaluation of TBI Patients
The initial approach includes a focused history and thorough physical examination. Physical examination should include a careful airway assessment as well as a thorough neurological examination to determine baseline sensation, motor function, and the presence of new focal neurological deficits to establish degree of traumatic brain injury or cervical spine injury severity [8]. During this first assessment it is important to recognize critical signs of other trauma related injuries such as bleeding, pneumothorax, cardiac tamponade, etc.
Recent studies showed that the protein High Mobility Group Box-1 (HMGB1) is released from damaged neurons into the cerebral spinal fluid and serum. This protein may serve as a prognostic biomarker for risk stratification and treatment of TBI patients in the future [9].
Cervical Spine Injury (CSI) after TBI
Early assessment of cervical spine integrity is essential to rule out a hidden cervical spine fracture, especially in the TBI patient. Demetriades, et al. found in his study, 292 cases of Cervical Spine Injury (CSI) amongst 14,755 TBI admissions (2%) [10]. Most importantly, this study showed a correlation between CSI and GCS. There was an increased incidence of CSI (10.2%) in patients with a GCS less than 8. Therefore, the clinician should maintain a high degree of suspicion for CSI in a TBI patient with a low GCS [10]. During physical examination of the cervical spine the clinician should pay close attention to findings such as tenderness along the spine, a "gap" or "step" deformity in the continuity of the spine, or other mass effect due to edema, hematoma, or muscle spasm [11].
Imaging in CSI Patients
An important step in improving efficiency in treating TBI patients involves appropriate utilization of imaging studies for detection of CSI. There are two well established guidelines for obtaining cervical spine radiographic imaging of TBI patients. The National Emergency X-Radiography Utilization Study (NEXUS) and the Canadian C Spine Rule. The NEXUS study concluded that a patient was at low risk for CSI if none of the five clinical criteria contained in Table 1 were present.
![]()
Table 1: A table of the NEXUS criteria for a patient at low risk of a cervical spine injury [12].
View Table 1
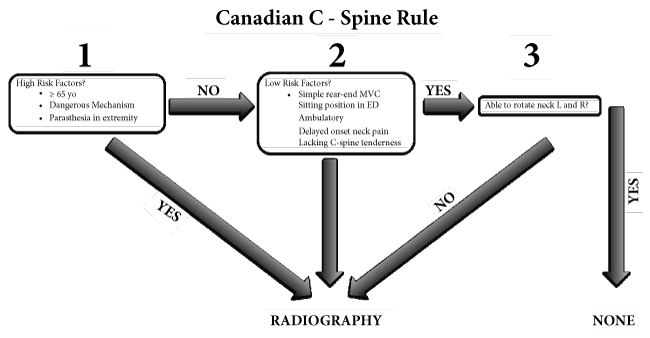
The NEXUS study correctly identified 810 of 818 patients with CSI from a sample of 34,069 patients (99% sensitivity, 12.9% specificity) [12]. Another study, the Canadian C-spine Rule for Radiography after Trauma involved a multi-center, prospective cohort of 8,294 patients with trauma to the head or neck to establish guidelines for ordering radiographic imaging. Major indications for imaging included age greater than 65, a dangerous mechanism of injury, a sensory neurological deficit, and the inability of the patients to rotate their necks forty-five degrees. The study indicators found a 100% sensitivity and 42.5% specificity [13], see Figure 1.
Stiell, et al. compared the NEXUS criteria to the Canadian C-spine Rule in 8,283 trauma patients. The results indicated that the Canadian C-spine Rule was more sensitive than the Nexus criteria showing a sensitivity of 99.4% vs. 90.7% respectively and a specificity of 45.1% vs. 36.8 respectively in the detection of CSI [14].
Once a clinician suspects CSI, the cervical spine should be immediately immobilized with a cervical collar [11]. Diaz JJ, et al. demonstrated in his study that Helical Computed Tomography (HCT) scan of the cervical spine was superior to plain radiography in the detection of cervical spine fractures. The study showed that plain radiography failed to detect cervical spine fractures in 208 from 278 patients suffering from cervical spine fracture, (74.8%) [11,15]. In the same study, they reported that if ligamentous injury of the cervical spine is suspected magnetic resonance imaging is superior to HCT [15].
Ideally, the radiographic images should be interpreted by a radiologist [11]. Following the recommendations by Diaz, et al. in the event that focal neurological deficits exist or another imaging modality shows significant findings, Magnetic Resonance Imaging (MRI) is warranted [15].
For traumatic brain injury patients requiring multiple surgical interventions the use of intraoperative CT imaging to identify residual or delayed hematoma has been associated with improved neurological outcomes at discharge when compared to fixed-unit CT [16].
Securing the Airway
In the event a radiographic study cannot be performed due to hemodynamic instability or airway emergency, all patients with severe trauma or head injuries should be assumed to have an unstable cervical spine until proven otherwise radiographycally.
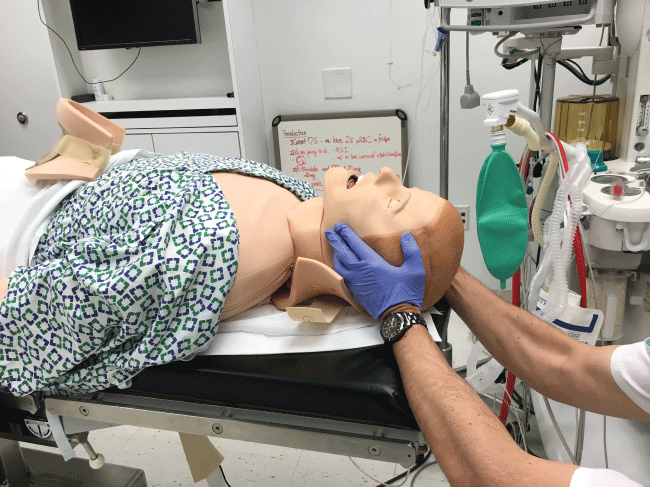
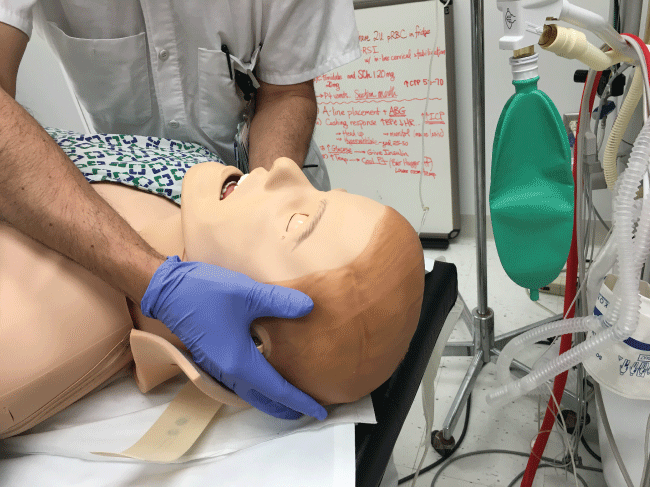
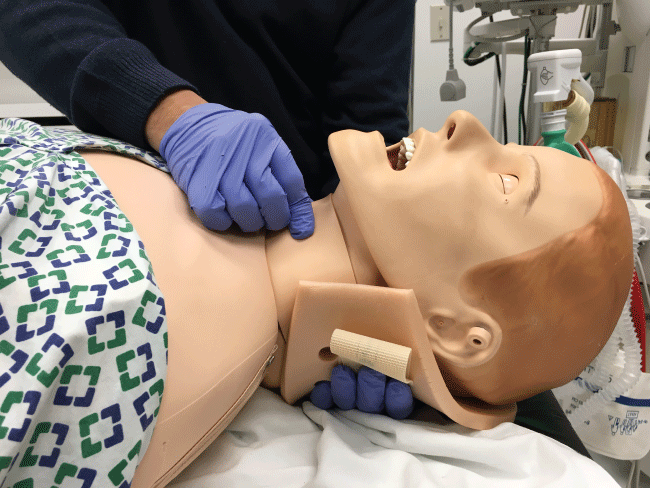
In the initial management of a TBI patient with possible CSI, prevention of further neurological injury is critical. Maintaining spinal alignment, protecting the spinal cord, and stabilizing the cervical spine can prevent further injury. The use of a cervical collar may stabilize the spine; however, its presence may interfere with direct laryngoscopy during intubation. Therefore, removal of only the anterior portion of the cervical collar is recommended, leaving the posterior portion in place, prior to attempt direct laryngoscopy and intubation [17,18]. To maintain the patient's cervical spine immobile during laryngoscopy several techniques have been developed. The most common is called Manual In-Line Immobilization (MILI). MILI is performed with the patient in supine position by an assistant, standing at the head of the bed, or by the patient's side. The technique consists of hand cradling the occiput and with the tip of the fingers stabilizing the mastoid process, see Figure 2. Alternatively, by standing at the side of a supine patient, an assistant can cradle the mastoid process and place his or her fingertips on the occiput, see Figure 3. Another maneuver that can be used in combination with MILI, consists of the application of cricoid pressure, plus removal of the anterior half of the cervical collar and manual posterior cervical spine support, see Figure 4. The use of MILI has less impact obtaining a view of the vocal cords during direct laryngoscopy compared to immobilization with axial traction utilizing a cervical collar, tape, or sandbags [18,19].
.
Figure 2: Manual In Line Immobilization (MILI) of the cervical spine Approach from the head of the bed.
View Figure 2
.
Figure 3: MILI of the cervical spine. Approach from the lateral side of the patient to facilitate airway management from the head of the bed.
View Figure 3
Historically, practitioners preferred nasotracheal intubation with flexible bronchoscopy or a surgical airway due to the possibility of spinal injury during oral intubation [20]. However, performing a nasotracheal intubation in patients with suspected basal skull fracture is a relative contraindication, because the endotracheal tube inserted blindly might find its path to the brain. Another complication from nasotracheal intubation is trauma to the nares and epistaxis. Presence of blood in the oropharynx from epistaxis will make visualization of the vocal cords extremely difficult not only when performing direct laryngoscopy but also while using videolaryngoscopes or fiberoptic bronchoscopes. Therefore, orotracheal intubation after rapid sequence inductionand direct laryngoscopy with MILI, remains the more expeditious safe choice to secure the airway in this group of patients. If airway management history or assessment reveals a possible challenging intubation, an awake fiberoptic intubation should be performed instead [17-20]. To the experienced practitioner, an awake fiberoptic approach is effective because it maintains the cervical spine in a neutral position, preserves airway reflexes, and allows simultaneous neurological assessments [21]. However, for practitioners inexperienced with the flexible bronchoscope, direct laryngoscopy with MILI may be a better option [22]. The use of a Miller, Macintosh, or McCoy blade has not shown statistically significant differences in outcomes when comparing spinal movement [23]. Time to intubation is longest with a video-laryngoscope compared to a Macintosh blade. Cervical spine movement is considered insignificant between the two [23]. Movement of the cervical spine during use of cricoid pressure to aid visualization of the vocal cords was of questionable significance in cadaveric studies. If cricoid pressure will be use, in addition to leaving the posterior portion if the cervical spine collar in place, manual posterior cervical spine support should be performed, see Figure 4 [20,24]. In some cases placement of a Laryngeal Mask Airway (LMA) must be needed to assure airway patency or to use it as a conduit for endotracheal intubation. In these cases the clinician must be aware that some studies have shown that during LMA insertion the downward pressure exerted could produce a potential displacement of the upper cervical spine [25]. Lastly, a surgical airway, cricothyrotomy, should be performed in the event of a can't intubate & can't ventilate scenario or if trauma to the upper airway makes intubation or ventilation impossible [20,26].
.
Figure 4: In addition to MILI, if the clinician is going to apply cricoid pressure, manual posterior cervical spine support is recommended to decrease cervical spine movement.
View Figure 4
Ventilation in TBI
Securing the airway by intubating our patient has three specific goals, prevention of aspiration of gastric contents and prevention of hypoxia and hypercarbia. In the presence of hypoxia or hypercarbia, cerebral veins dilate, causing an increase in ICP. Tissue hypoxia leads to release of catecholamines which further dilate cerebral veins and increase ICP [27,28]. Additionally, the presence of hypoxia has direct correlation to poor outcomesin TBI patients [29,30]. A Level 3 recommendation by the BTF is to avoid a PaO2 less than 60 mmHg and to maintain an oxygen saturation greater than 90% [4]. In the event, that signs and symptoms of elevated ICP such as, new onset of focal neurological deficits, decreased consciousness, inappropriate pupillary responses, vomiting, cardiac or respiratory arrest, or Cushing's triad of hypertension, bradycardia, and bradypnea, or signs of brain herniation are present, hyperventilation should be performed [4,30]. Hyperventilation is a level 3 recommendation by the BTF as a temporizing measure to reduce ICP. The clinician should be aware that excessive hyperventilation can lead to cerebral vasoconstriction and oxygen deprivation [4].
Preoperative Management
The clinician should avoid hypercarbia related to the administration of hypnotic agents or sedatives such as benzodiacepines, narcotics, etc, prior to induction of anesthesia.
Intraoperative Management
The choice and dose of anesthetic agents to provide anesthesia and hence avoid hypotension have an important impact in TBI patients' outcome.
Choice of Anesthetic Drugs
Muscle relaxants
As mentioned before, a rapid sequence induction is commonly performed in this group of patients. Succinylcholine, is the neuromuscular blocking agent of choice for this purpose. Minton, et al. found that after its administration patients developed a transient increase in ICP [31]. Despite this potential side effect, the benefit of its rapid onset and duration of action and the prevention of coughing during direct laryngoscopy greatly outweighs its negative effect. In addition Stirt, et al. described a way to prevent this side effect by administering a defasciculating dose of a non-depolarizing muscle relaxant [31,32]. If a clinician decides to avoid succinylcholine, then the administration of rocuronium 0.9-1.2 mg/kg will achieve same intubating conditions like succinylcholine at 60-90 seconds, with this drug there is no transient increase in ICP however muscle paralysis might last for 30 to 40 minutes [33].
Recently the thought that ketamine had a deleterious effect in patients with increased ICP was challenged [34]. Early studies concluding that ketamine increased CBF and ICP were based on small sample sizes with higher than recommended induction doses. Recent studies do not show an increase in ICP after its administration [34]. The entire contrary, they reported potential benefits with the use of ketamine including, the blockade of reuptake of catecholamines, which can prevent hypotensive episodes by maintaining mean arterial pressure and cerebral perfusion pressure within normal range. Logically ketamine should be avoided in hypertensive TBI patients due to the risk of further elevating blood pressure and consequently increasing ICP. In TBI patients with suspected elevated ICP and low-to-normal blood pressure, ketamine use might be indicated to preserve normotension during induction of anesthesia [34,35]. Etomidate is another appropriate induction agent choice in hemodynamically unstable patients. It is important to remember that etomidate may cause a dose dependent inhibition of 11-beta-hydroxylase and 17-alpha-hydroxylase leading to adrenal suppression. This complication can occur after a single dose and may cause maximal adrenal suppression 4 to 6 hours after its administration. No study today has shown an increase in mortality after its administration [36].
Propofol is indicated as a sedative agent in the TBI patient with a secure airway. This intravenous anesthetic has the advantage of a quick onset and offset of action that facilitates neurologic assessment. Propofol might be beneficial in this group of patients, because it might decrease neuronal oxidative stress. The clinician should be aware of propofol's sympathetic blockade resulting in hypotension. Hypotension regardless of the cause should be addressed promptly since it is very poorly tolerated by this group of patients and affects their outcomes [4,37]. Another complication that may arise from the use of propofol is a condition known as propofol infusion syndrome. This condition occurs generally if propofol is delivered for more than 48 hours, at doses above 4 mg/kg/hr [37].
Additionally, propofol may be indicated in the treatment of refractory status epilepticus with a recommended starting loading dose of 1 mg/kg [38-40].
Opioids are used during induction of anesthesia to suppress airway reflexes, decrease required dose of induction agents and inhalation anesthetic maintenance as well as to blunt the sympathetic response to direct laryngoscopy. Fentanyl, sufentanil, and remifentanil are commonly used in TBI patients. Careful opioid titration should be observed to avoid hypotension secondary to a reduction in sympathetic tone and potential histamine release from these agents [41].
Maintenance of anesthesia
During maintenance of anesthesia, intravenous and inhalation anesthetics can be used safely in the care of TBI patients while observing the guidelines set by the BTF. To achieve this goal we must have an understanding of their effects on the brain vasculature and metabolism. Intravenous anesthetics such as sodium thiopental, etomidate, midazolam and propofol decrease Cerebral Blood Flow (CBF), Cerebral Blood Volume (CBV), Cerebral Metabolic Rate (CMRO2) and Intracranial Pressure (ICP) under controlled ventilation conditions. They achieve these effects by producing cerebral vasoconstriction and acting at the neurons' GABA receptors to open chloride channels [42,43]. Regarding dexmedetomidine, an alpha 2 receptor agonist, it exerts its effects in the locus coeruleus. Despite its sedative and anxiolytic action it preserves adequate respiratory function when compared with benzodiacepines or narcotics. This property makes it an ideal agent in the non intubated TBI patient. In the Intensive Care Unit (ICU) setting dexemedetomidine has proved to decrease the incidence of delirium. Further research is needed to determine the impact of dexemedetomidine in the outcomes of TBI patients, however the data presented makes it suitable alternative to propofol for sedation purposes [37].
In term of narcotics, in the review article by Schregel W, et al. to determine the effects of alfentanil, fentanyl and sufentanil on CBF and ICP [41]. He found that after the administration of an intravenous bolus of narcotics in patients with brain injury and elevated ICP, opioids did not further increase ICP despite a transient decrease in mean arterial pressure [41]. The use of opioids is justified to blunt the sympathetic response during intubation and surgical stimulation and to avoid a hypertensive response that would further increase ICP.
Inhalation Anesthetics (IAs) produce a dose dependent increase in CBF called "uncoupling effect" that may lead to increase ICP [42]. This effect can be avoided by titrating IAs to levels below 1 Minimal Alveolar Concentration (MAC) [42-44]. While nitrous oxide is the only inhalation anesthetic that produces an increase in CMRO2, the rest, isoflurane, sevoflurane and desflurane decrease CMRO2 [44-46]. Therefore, the use of nitrous oxide should be avoided in TBI patients. The literature currently finds no difference in outcomes between the use of inhalation anesthetics and intravenous anesthetics or the combination of both in the intraoperative and perioperative care of TBI patients [42-46]. We consider pertinent to highlight again that there is no ideal anesthetic, instead the anesthesiology team should follow the BTF clinical guidelines while administering anesthesia to this group of patients, especially avoiding hypoxemia, PaO2 below 60 mmHg, oxygen saturation below 90%, hypercarbia and hypotension, (systolic blood pressure below 90 mmHg) [4].
Intraoperative monitoring and intravenous access
Besides standard ASA monitors, an arterial line and adequate intravenous access are essential in the management of TBI patients. One very important thing to consider is that placement of these lines should not delay the start of the surgical intervention. Placement of two large bore (greater than or equal to 18 gauge) intravenous access sites and invasive hemodynamic monitoring is recommended. If the patient has difficult intravenous access and requires a central line, the femoral vein is most appropriate in order to avoid trendelenburg positioning, which may lead to increased intracranial pressure [47,48]. If peripheral and central line placement fail, then tibial or humeral intra-osseous lines should be placed.
Blood pressure management
Neuronal death after TBI may have different etiologies. It can be directly related to the primary injury or related to secondary injuries such as brain hypoperfusion and hypoxemia among others. The BTF has found that a single pre-hospital observation of hypotension (Systolic blood pressure < 90 mmHg) was a statistically independent risk factor for poor outcome in TBI patients [4]. The other major outcome predictors described by the BTF were age, glasgow coma scale on admission, intracranial pathology, and pupillary status [4,49].
When addressing the question of what's the recommended blood pressure range in this group of patients, it is essential to know what are the determinants of Cerebral Perfusion Pressure (CPP). Cerebral Perfusion Pressure (CPP) = Mean Arterial Pressure (MAP)-Intracranial Pressure (ICP) or Central Venous Pressure (CVP) depending on which one is the highest. This equation highlights the direct relation between cerebral perfusion and systemic blood pressure. If CPP decreases, brain parenchyma oxygenation can be further compromised in TBI patients.
The BTF is basing their recommendation of maintaining these patients systemic blood pressure above 90 mmHg in the findings by Chestnut RM, et al. They described an incidence of hypotension in TBI patients of around 34.6%, but what was of great concern is that in this subset of patients there was a 150% mortality increased [50]. Yet another important finding in TBI patients described by Bouma, et al. is the fact that 30% of TBI patients experience oligemic Cerebral Blood Flow (CBF) as well as ischemic venous jugular oximetry related to increased ICP within the first 6 hours after TBI. Therefore treatment should not only focus in keeping CPP within normal range but also in decreasing ICP [51]. Trying to address the specific CPP range to optimize TBI patient outcomes, we have to mention the studies by Chan, et al. and Robertson, et al.
Chan, et al. demonstrated that a reduction in CPP to less than 70 mmHg, produces a decline in jugular venous oxygen saturation and an increase in transcranial doppler ultrasonography pulsatility index in TBI patients [52]. Robertson, et al. compared the outcomes of 2 groups of TBI patients with different goals of CPP. In the first group CPP was kept above 70 mmHg in the second group CPP was maintained between 50 mmHg and 70 mmHg. The study showed no difference in neurological outcome, however, the group of patients were CPP was kept above 70 mmHg had a five-fold increase in pulmonary edema and Adult Respiratory Distress Syndrome (ARDS) (15% vs. 3%) [49].
Therefore, the current recommendations are to keep the systolic blood pressure above 90 mmHg and the CPP between 50 and 70 mm Hg to avoid further brain ischemia [4,49,50].
Management of hypotension with vasopressor is common. The choice of which vasopressor to use is less clear. Sookplung, et al. examined patients with severe TBI who received phenylephrine, norepinephrine, or dopamine. Based on this study, phenylephrine had the greatest increase in MAP and CPP. The study concluded that it was unclear whether the improved MAP and CPP improved CBF and oxygenation [53]. In conclusion, the best choice of vasopressor for patients with TBI remains unclear.
Conversely, the ideal medication for treatment of hypertension in this group of patients, should be one that is easily titratable and should not cause cerebral vasodilatation such asnitroglycerine, nitroprusside, and hydralazine to avoid further increase in ICP. Therefore the antihypertensive drugs recommended include propranolol, esmolol, labetalol, and nicardipine [54].
Management of ICP
The Brain Trauma Foundation states that ICP > 20 mmHg is associated with increased mortality and worse outcomes [4]. The fastest way to decrease ICP > 20 mmHg is to allow Cerebrospinal Fluid (CSF) drainage from a CSF drain if present. Another relatively quick and effective alternative is to elevate the patient's head and maintain the neck in a neutral position, to improve venous blood return. Less rapid methods include slow administration of 0.25-1 gm/kg of mannitol in stable patients over fifteen minutes [4,55]. This can result in an ICP reduction, a transient increase in oxygen transport, and an increase in cerebral blood flow. Additional dosing at a rate of 0.25-0.5 gm/kg can be repeated every six to eight hours. Importantly, when using mannitol, it is important to monitor and replace urinary loses to prevent intravascular volume depletion and hypotension [55].
Alternatively, hyperventilation can temporarily treat intracranial hypertension, but should be used with caution. While studies have shown hyperventilation reduces ICP, it can also decrease brain oxygenation leading to adverse outcomes. Therefore it is recommended to use it with caution and as a temporary measure. Maintaining a normal PaCO2 of 35-40 mmHg is recommended in TBI patients to improve cerebral perfusion unless signs and symptoms of increased ICP are present [4].
Another modality to treat increased ICP is hypertonic saline, which provides osmotic mobilization of water across the intact blood brain barrier leading to a reduction in cerebral water content. This can decrease the ICP and improve blood flow to the brain. Multiple prospective observational studies have shown an average reduction in ICP ranging from 20-60% with time to peak effect range between 10 minutes and 5 hours post infusion [4,56]. Close attention to blood sodium levels is imperative to prevent hypernatremia. Ideally, blood sodium should be maintained not higher than 150-155 mEq/ml with a blood osmolality of less than 320 mOsm/dl. Hypertonic saline has the benefit of not causing hypotension as compared to the use of mannitol [4,56].
Hypotonic solutions are contraindicated because they add free water that might lead to cerebral edema and worsened ICP in a TBI patient [57]. Therefore isotonic solutions should be used for fluid resuscitation in TBI patients. 0.9% normal saline solutions are indicated because they are more isotonicthan Ringer's lactate. Glucose containing solutions should be avoided, unless hypoglycemia is present. We have to mention the SAFE study when recommending best fluid type (crystalloid vs. colloid) for volume resuscitation of the TBI patient. In this study TBI patients were randomized in 2 groups normal saline and albumin. The authors found that fluid resuscitation with albumin was associated with a higher mortality as compared with patients receiving normal saline (33% vs. 20%). This risk was even more pronounced in those with severe TBI (42% vs. 22%). Therefore, the current recommendation for TBI patients is to use normal saline [58].
Coagulopathy and hemoglobin level
TBI may produce coagulopathy through the systemic release of by-products from neuronal death such as tissue factor and phospholipids leading to disseminated intravascular coagulation. Therefore, coagulation parameters should be measured immediately in acute TBI patients [59]. Any abnormal values should be identified and corrected. INR in TBI patients should be maintained less than or equal to 1.4 and the platelet count maintained above 75 k/uL [4,60].
Hemoglobin levels should be maintained at or above 7 g/dl to avoid a decrease in brain oxygen delivery. It is important to mention that currently there is no evidence to support improvement in outcomes with aggressive transfusion therapy, to a hemoglobin level of 10 g/dl [4,60]. Robertson, et al. reported no neurological improvement after traumatic brain injury at six months when utilizing erythropoietin and transfusion thresholds of 10 g/dl versus 7 g/dl. Additionally, the risk for transfusion related reactions and infection increased with more liberal transfusion parameters; thus, treatment should be guided by clinical judgment and blood transfusion should occur if hemoglobin levels reach values below 7 g/dl [61,62].
Glycemic control
The presence of hyperglycemia might produce an increase in neuronal metabolism and increase neuronal death after TBI. These events occur due to increased tissue acidosis through anaerobic metabolism, the creation of free radicals, and increased blood brain barrier permeability. Therefore the ideal blood glucose level should range from 80-180mg/dl [63].
Thermoregulation
Lastly, even though multiple animal studies have shown an improved outcome when hypothermia is used after TBI. Human studies unfortunately, have not shown these same results. Clifton, et al. showed no benefit of induced hypothermia on mortality or neurological outcomes after TBI [4,64].
When referring to hyperthermia instead, it is important to remember that fever worsens the severity of brain injury by increasing cerebral metabolic rate [4]. In addition, early hyperthermia after TBI has been found to be a possible predictor of paroxysmal sympathetic hyperactivity by Hinson HE, et al. [65].
The final BTF recommendation is to avoid hyperthermia and to maintain normothermia with antipyretics and surface cooling devices [4].
Conclusion
Managing TBI patients in the perioperative setting is a challenging task. Guidelines developed over the past few decades have had a significant effect on decreasing mortality and improving outcome. Recognizing the potential for cervical spine injury along with the determination for the need of imaging studies is essential while treating a TBI patient. Rapid sequence induction and direct laryngoscopy with the application of MILI, continues to be the fastest way to secure these patients' airway in the emergency. Avoiding hypocarbia along with hypoxia can attenuate cerebral vasodilation and catecholamine surge. The anesthesiologist should be aware about the pharmacokinetic and pharmacodynamic properties of the agents that are going to be used and the anesthetic goal should be to keep the TBI patient normotensive. Large bore intravenous access is recommended in order to be prepared to correct significant fluid shifts along with the possible need to infuse vasopressor. Hypotension and hypoxia have been shown to be independent risk factors for increased mortality and should be treated promptly. To maintain oxygen delivery to the brain, the hemoglobin level should be kept at or above 7 g/dL. If ICP is elevated, temporary ways to decrease ICP include elevation of the patient's head, hyperventilation, administration of diuretics, switching inhalation anesthesia to total intravenous anesthesia, use of hypertonic saline solutions, and if neurosurgical indicated, establishing a more definitive treatment by placing an external ventricular device to drain cerebrospinal fluid and to monitor ICP, or to proceed with the performance of a decompressive craniotomy.
References
-
León-Carrión J, del Rosario Domínguez-Morales M, y Martín JMB, Murillo-Cabezas F (2005) Epidemiology of traumatic brain injury and subarachnoid hemorrhage. Pituitary 8: 197-202.
-
Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States. Atlanta, GA Centers Dis Control Prev Natl Cent Inj Prev Control.
-
Bratton SL, Chestnut RM, Ghajar J, McConnell HFF, Harris OA, et al. (2006) Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma 24: S7-S13.
-
(2007) Guidelines for the Management of Severe Traumatic Brain Injury 3rd Edition. Brain Trauma Foundation, American Association of Neurological Surgeons (AANS) Congress of Neurological Surgeons, Joint section on Neurotrauma and Critical Care. Journal of Neurotrauma 24.
-
Reid P, Butler D, Buono J, Erdtmann F (2009) Systems Engineering to Improve Traumatic Brain Injury Care in the Military Health System: Workshop Summary. National Academies Press.
-
Greve MW, Zink BJ (2009) Pathophysiology of traumatic brain injury. Mt Sinai J Med 76: 97-104.
-
Curry P, Viernes D, Sharma D (2011) Perioperative management of traumatic brain injury. Int J Crit Illn Inj Sci 1: 27-35.
-
Ackland H, Cameron P (2012) Cervical spine: assessment following trauma. Aust Fam Physician 41: 196-201.
-
Parker TM, Nguyen AH, Rabang JR, Patil A-A, Agrawal DK (2016) The danger zone: Systematic review of the role of HMGB1 danger signalling in traumatic brain injury. Brain Inj 31: 2-8.
-
Demetriades D, Charalambides K, Chahwan S, Hanpeter D, Alo K, et al. (2000) Nonskeletal cervical spine injuries: epidemiology and diagnostic pitfalls. J Trauma Inj Infect Crit Care 48: 724-727.
-
Crosby ET (2006) Airway management in adults after cervical spine trauma. J Am Soc Anesthesiol 104: 1293-1318.
-
Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI (2000) Validity of a set of clinical criteria to rule out injuryto the cervical spine in patients with blunt trauma. N Engl J Med 343: 94-99.
-
Stiell IG, Wells GA, Vandemheen KL, Clement CM, Lesiuk H, et al. (2001) The Canadian C-Spine rule for radiography in alert and stable trauma patients. JAMA 286: 1841-1848.
-
Stiell IG, Clement CM, McKnight RD, Brison R, Schull MJ, et al. (2003) The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med 349: 2510-2518.
-
Diaz Jr JJ, Aulino JM, Collier B, Roman C, May AK, et al. (2005) The early work-up for isolated ligamentous injury of the cervical spine: does computed tomography scan have a role? J Trauma Acute Care Surg 59: 897-904.
-
Chen KT, Lee ST, Wu CT (2017) The Clinical Value of Intraoperative Mobile Computed Tomography in Managing High-Risk Surgical Patients with Traumatic Brain Injury-A Single Tertiary Trauma Center Experience. World Neurosurg 98: 727-733.
-
Heath KJ (1994) The effect on laryngoscopy of different cervical spine immobilization techniques. Anaesthesia 49: 843-845.
-
Austin N, Krishnamoorthy V, Dagal A (2014) Airway management in cervical spine injury Int J Crit Illn Inj Sci 4: 50-56.
-
Walls RM (1987) Orotracheal intubation and potential cervical spine injury. Ann Emerg Med 16: 373.
-
Rosenblatt WH, Wagner PJ, Ovassapian A, Kain ZN (1998) Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg 87: 153-157.
-
Chesnut RM (2004) Management of brain and spine injuries. Crit Care Clin 20: 25-55.
-
Khan FA (2014) Airway Management in Traumatic Brain Injury (TBI). In: Airway Management. Springer, 147-156.
-
Gerling MC, Davis DP, Hamilton RS, Morris GF, Vilke GM, et al. (2000) Effects of cervical spine immobilization technique and laryngoscope blade selection on an unstable cervical spine in a cadaver model of intubation. Ann Emerg Med 36: 293-300.
-
Turkstra TP, Craen RA, Pelz DM, Gelb AW (2005) Cervical spine motion: a fluoroscopic comparison during intubation with lighted stylet, GlideScope, and Macintosh laryngoscope. Anesth Analg 101: 910-915.
-
Keller C, Brimacombe J, Keller K (1999) Pressures Exerted Against the Cervical Vertebrae by the Standard and Intubating Laryngeal Mask Airways: A Randomized, Controlled, Cross-Over Study in Fresh Cadavers. Anesth Analg 89: 1296-1300.
-
Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, et al. (2013) Practice guidelines for Management of the Difficult Airway: An Updated Report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 118: 251-270.
-
Van Loon J, Shivalkar B, Plets C, Goffin J, Tjandra-Maga TB, et al. (1993) Catecholamine response to a gradual increase of intracranial pressure. J Neurosurg 79: 705-709.
-
Ogilvy CS, DuBois AB (1987) Effect of increased intracranial pressure on blood pressure, heart rate, respiration and catecholamine levels in neonatal and adult rabbits. Neonatology 52: 327-336.
-
McHugh GS, Engel DC, Butcher I, Steyerberg EW, Lu J, et al. (2007) Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma 24: 287-293.
-
Griesdale DE, Örtenwall V, Norena M, Wong H, Sekhon MS, et al. (2015) Adherence to guidelines for management of cerebral perfusion pressure and outcome in patients who have severe traumatic brain injury. J Crit Care 30: 111-115.
-
Minton MD, Grosslight K, Stirt JA, Bedford RF (1986) Increases in intracranial pressure from succinylcholine: Prevention by prior nondepolarizing blockade. Anesthesiology 65: 165-169.
-
Stirt JA, Grosslight K, Bedford RF, Vollmer D (1987) "Defasciculation" with metocurine prevents succinylcholine induced increases in intracranial pressure. Anesthesiology 67: 50-53.
-
Neuromuscular blocking agents. (5th edn), Morgan& Mikhail's Clinical Anesthesiology, Rocuronium.
-
Zeiler FA, Teitelbaum J, West M, Gillman LM (2014) The ketamine effect on ICP in traumatic brain injury. Neurocrit Care 21: 163-173.
-
Wang X, Ding X, Tong Y, Zong J, Zhao X, et al. (2014) Ketamine does not increase intracranial pressure compared with opioids: meta-analysis of randomized control trials. J Anesth 28: 821-827.
-
Dearden NM, McDowall DG (1985) Comparison of etomidate and althesin in the reduction of increased intracranial pressure after head injury. Br J Anaesth 57: 361-368.
-
Flower O, Hellings S (2012) Sedation in Traumatic Brain Injury. Emerg Med Int 2012: 637171.
-
Claassen J, Hirsch LJ, Emerson RG, Mayer SA (2002) Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 43:146-153.
-
Miller RD (2009) Miller's Anesthesia. Elsevier Saunders.
-
Turner BK, Wakim JH, Secrest J, Zachary R (2005) Neuroprotective effects of thiopental, propofol and etomidate. AANA J 73: 297-302.
-
Schregel W, Weyerer W, Cunitz G (1994) Opioids, cerebral circulation and intracranial pressure. Anaesthesist 43: 421-430.
-
Engelhard K, Werner C (2006) Inhalation or intravenous anesthetics for craniotomies? Proinhalational. Curr Opin Anaesthesiol 19: 504-508.
-
Kaisti KK, Långsjö JW, Aalto S, Oikonen V, Sipilä H, et al. (2003) Effects of sevoflurane, propofol and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99: 603-613.
-
Grathwohl KW, Black IH, Spinella PC, Sweeney J, Robalino J, et al. (2008) Total intravenous anesthesia including ketamine versus volatile gas anesthesia for combat-related operative traumatic brain injury. Anesthesiology 109: 44-53.
-
http://www.medicalgasresearch.com/content/2/1/10.
-
Zuleta-Alarcon A, Castellón-Larios K, Ni ño-de Mejía MC, Bergese SD (2015) Total intravenous anaesthesia versus inhaled anaesthetics in neurosurgery. Rev Colomb Anesthetsiol 43: 9-14.
-
Halverson A, Buchanan R, Jacobs L, Shayani V, Hunt T, et al. (1998) Evaluation of mechanism of increased intracranial pressure with insufflation. Surg Endosc 12: 266-269.
-
Rosenthal RJ, Hiatt JR, Phillips EH, Hewitt W, Demetriou AA, et al. (1997) Intracranial pressure. Effects of pneumoperitoneum in a large-animal model. Surg Endosc 11: 376-380.
-
Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, et al. (1999) Prevention of secondary ischemic insults after severe head injury. Crit Care Med 27: 2086-2095.
-
Chestnut, Marshall LF, Klauber MR, Blunt BA, Baldwin N, et al. (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34: 216-222.
-
Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF (1991) Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 75: 685-693.
-
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S (1992) The effect of changes in cerebral perfusion pressure upo middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurgery 77: 55-61.
-
Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang J, et al. (2011) Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care 15: 46-54.
-
Robertson CS, Clifton GL, Taylor AA, Grossman RG (1983) Treatment of hypertension associated with head injury. J Neurosurgery 59: 455-460.
-
Muizelaar JP, Lutz III HA, Becker DP (1984) Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J Neurosurg 61: 700-706.
-
Mortazavi MM, Romeo AK, Deep A, Griessenauer CJ, Shoja MM, et al. (2012) Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis: a review. J Neurosurg 116: 210-221.
-
Zander R (2009) Intracranial pressure and hypotonic infusions solutions. Anaesthetsist 58: 405-409.
-
Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, et al. (2007) Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 357: 874-884.
-
Zehtabchi S, Soghoian S, Liu Y, Carmody K, Shah L, et al. (2008) The association of coagulopathy and traumatic brain injury in patients with isolated head injury. Resuscitation 76: 52-56.
-
Reddy GD, Gopinath S, Robertson C (2016) Critical Care Management of the Patient with Traumatic Brain Injury. Semin Neurol 36: 570-576.
-
Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, et al. (2009) The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med 37: 1074-1078.
-
Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, et al. (2014) Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. Jama 312: 36-47.
-
Jeremitsky E, Omert L, Dunham C, Wilberger J, Rodriguez A (2005) The impact of hyperglycemia on patients with severe brain injury. J Trauma 58: 47-50.
-
Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, et al. (2011) Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol 10: 131-139.
-
Hinson HE, Schreiber MA, Laurie AL, Baguley IJ, Bourdette D, et al. (2017) Early fever as a predictor of paroxysmal sympathetic hyperactivity in traumatic brain injury. J Head Trauma Rehabil .