Clinical Medical
Reviews and Case Reports
An Ileal Perforation Associated with Extended Spectrum β-Lactamases-Producing Escherichia Coli: Medical Case Report
Michael Owusu1, KojoSarfo Marfo1, Isaac Osei1, John Amuasi4, Nimako Sarpong2, Kofi Tawiah Mensah2, AugustinaAnnan1, Ellis Owusu-Dabo4*, Yaw Adu-Sarkodie3
1Kumasi Centre for Collaborative Research in Tropical Medicine, Kwame Nkrumah University of Science and Technology, Ghana
2Agogo Presbyterian Hospital, Ashanti Region, Ghana
3Department of Clinical Microbiology, Kwame Nkrumah University of Science and Technology, Ghana
4Department of Global Health, School of Public Health, Kwame Nkrumah University of Science and Technology, Ghana
*Corresponding author: Ellis Owusu-Dabo, Department of Global Health, School of Public Health, Kwame Nkrumah University of Science and Technology, Ghana, E-mail: owusudabo@kccr.de
Clin Med Rev Case Rep, CMRCR-3-140, (Volume 3, Issue 11), Case Report; ISSN: 2378-3656
Received: September 27, 2016 | Accepted: November 14, 2016 | Published: November 16, 2016
Citation: Owusu M, Marfo K, Osei I, Amuasi J, Sarpong N, et al. (2016) An Ileal Perforation Associated with Extended Spectrum β-Lactamases-Producing EscherichiaColi: Medical Case Report. Clin Med Rev Case Rep 3:140. 10.23937/2378-3656/1410140
Copyright: © 2016 Owusu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Intestinal perforation is one of the leading fatal causes of death among individuals mostly in developing countries. Although many reports have associated perforations with typhoid infections, reports on the role of other bacterial pathogens especially resistant strains in causing ileal perforations are limited. We report a rare case of ileal perforation associated with extended-spectrum beta-lactamase (ESBL) - producing Escherichia coli (E. coli) in Ghana.
Case presentation: A 9-year-old female child presented to the Agogo Presbyterian Hospital with a case of worsening abdominal pain and generalised abdominal tenderness. She was found to have ileal perforation upon surgical operation and her blood culture and swabs from the perforated sites all yielded E.coli which was resistant to all routine antibiotics except meropenem. Molecular and phenotypic analysis showed that the isolated bacteria had blaCTX-M and blaTEM- associated resistance genes. The patient responded well with meropenem administration and was discharged upon full recovery.
Conclusion: ESBL producing bacteria could be associated with ileal perforation in children. Physicians should be mindful of this and administer evidence based therapy when encountered with similar situation.
Keywords
ESBL, Ileal perforation, Resistance, Antimicrobial, Infections
List of Abbreviations
ESBL: Extended spectrum β-lactamase
Background
Gastrointestinal perforation occurs when a hole forms all the way through the stomach, large bowel, or small intestine as a result of trauma, appendicitis, foreign bodies, parasitic infections, tuberculosis and tumours [1-4]. The ileum is one of the commonest sites for perforations and has been associated with typhoid disease [5]. The infection occurs when the typhoid bacterium accesses the human host through ingested food or drink and infects the Peyer's patches in the ileum leading to necrosis, ulcerations and perforations. Complications associated with perforations are usually fatal and could result in death if early surgical intervention is not offered. Treatment is often dependent on resuscitation with fluids, electrolytes and administration of broad spectrum antibiotics such as quinolones, third generation cephalosporins and metronidazole.
Although many reports, especially in developing countries, have associated perforations with typhoid infections, reports on the role of other bacterial pathogens, especially resistant strains, in causing ileal perforations are limited. Resistant bacterial strains associated with intestinal perforation are the one of the most life threatening conditions which could often result in death if the appropriate antibiotics are not administered. A group of resistant bacteria which seem to be recognized in causing severe disease are the extended spectrum beta lactamase (ESBL) - producing bacteria (ESBL), of which E.coli is a member. The emergence of ESBLs followed the introduction into clinical practice of expanded-spectrum cephalosporins, which are acknowledged to be the most powerful selectors for these resistance determinants. Extended-spectrum beta-lactamase (ESBL) - producing E.coli cause a significant therapeutic challenge since they hydrolyze all β-lactams except carbapenems and cephamycins. Since 1960 when the first mutant form (TEM) was isolated, novel resistant genes including SHV, CTX-M and OXA families have been found [6-9].
In Ghana and in many other African countries, most reported ileal perforation cases are managed as typhoid and hence treated with β-lactam antibiotics and quinolones as part of routine patient care. Here, we report a rare case of ileal perforation associated with blaCTX-M- and blaTEM-β-lactamase Escherichia coli.
Case Presentation
A nine-year-old-female was referred from a rural health centre to the Agogo Presbyterian Hospital in the Asante Akim North district of the Ashanti region as a case of worsening abdominal pain with a 10-day history of fever. Her referred medical record showed she had severe abdominal pains 5 days before and was being treated with intravenous (IV) ciprofloxacin and metronidazole. On examination, the patient was pale, looked very ill and was thus haemo-transfused with 1 unit of blood before being referred to the Agogo Presbytarian Hospital (APH).
Her respiratory rate was 36 cycles per minute with a reduced bilateral air entry. The first and second heart sounds, blood pressure and pulse rate were all normal. There was, however, generalised abdominal tenderness, guarding and rebound tenderness. A preliminary diagnosis of generalised peritonitis secondary to perforated hollow viscus was made with differential diagnosis of ruptured appendicitis and typhoid ileal perforation. The patient was admitted at the intensive care unit and investigations including full blood count, liver function test, malaria test, sickling and chest x-ray were requested. Blood for culture and susceptibility testing was also collected and transported to the Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) for analysis. The patient was further administered with IV ciprofloxacin 300 mg twice daily for 72 hours and IV metronidazole 250 mg three times daily for 72 hours.
A kidney challenge with 500 ml of ringers lactate and 500 ml of normal saline was given over 30 mins in addition to 30 mg of IV Lasix. The urine output was assessed to monitor adequacy of the kidneys and the patient was prepared for laparotomy.
Laparotomy was performed on the 2nd day of admission and showed an ileal perforation at about 30 cm from the ileocecal junction, respectively. Both perforations occurred along the anti-mesenteric border. The diameter of the ileal perforation was 7 mm. The feculent peritoneal fluid was suctioned and the ileal perforation was sutured with vicry l2.0. Two swab samples were taken at the perforated site. Swab 1 was collected at the perforated site and swab 2 was collected a day after laparotomy at the sutured site. Both samples were transported to the bacteriology laboratory of KCCR for culture and antimicrobial susceptibility testing.
The vitals were monitored and metronidazole was replaced by clindamycin 300 mg three times daily for 7 days and temperature monitored for stability and improvement. The patient was still unwell two days after laparotomy and showing spiky temperature with respiratory distress. The IV ciprofloxacin was replaced with IV ceftriaxone 2 g daily for 72 hours; however the clinical condition of the patient did not improve.
On the 4th day of admission, the blood culture result was communicated to the hospital and the attending physician. The isolated bacterium was an ESBL producing E.coli which showed resistance to all antimicrobials except meropenem. The physician was therefore advised to administer meropenem to the patient. Intravenous meropenem 500 mg three times daily for 7 days was administered, and the patient's condition thereafter improved clinically and she was discharged upon full recovery. Stool samples were collected from the patient three days after discharge and sent to KCCR for culture and antimicrobial susceptibility testing. Figure 1 describes patient's response to antibiotic administration.
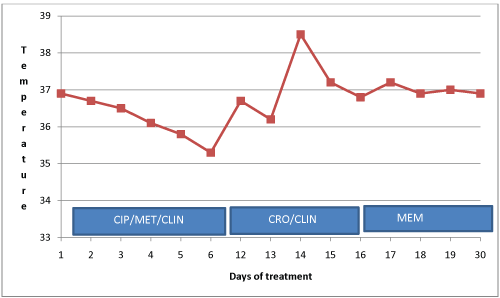
.
Figure 1: Response to antibiotic administration. CIP: Ciprofloxacin; CRO: Ceftriaxone; MET: Metronidazole; CLIN: Clindamycin; MEM: Meropenem.
View Figure 1
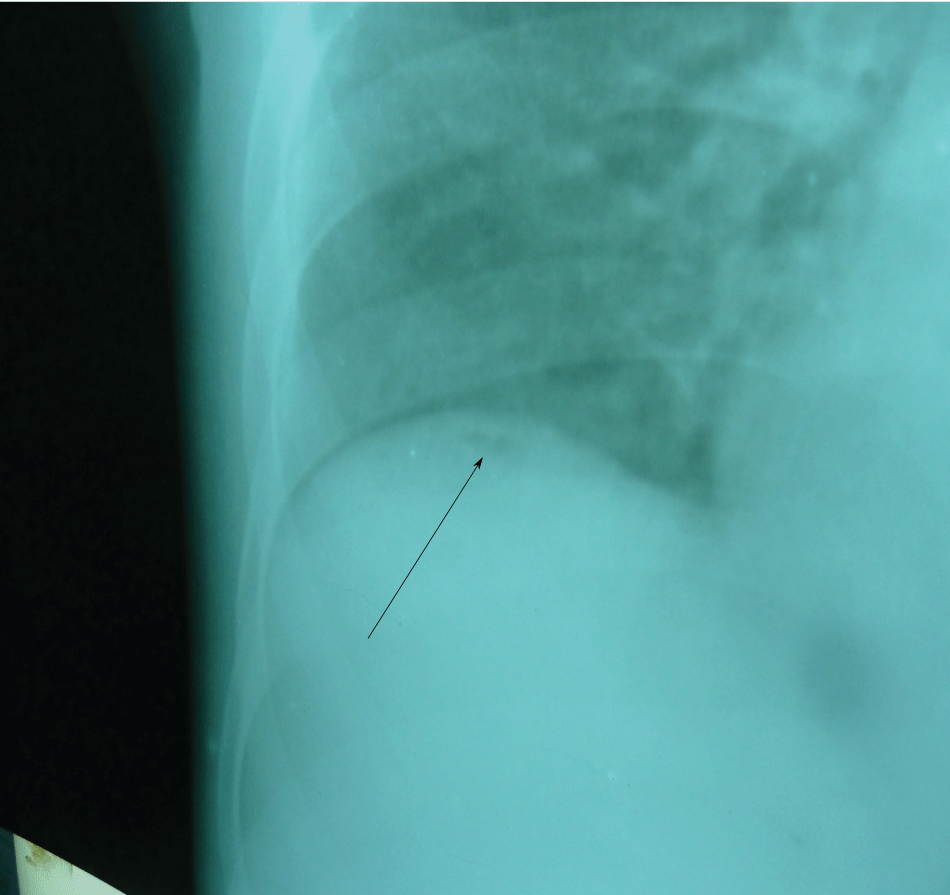
.
Figure 2: The right side of the chest X-ray showing small air bubble (arrow) below the right hemidiaphragm.
View Figure 2
Diagnostic Investigations
Basic investigations
Baseline full blood count investigations showed a haemoglobin concentration of 11.3 g/dl, total white blood cell count of 6900 cells/μl (lymphocyte 11.5%, neutrophil 80.3% and mixed cells of 8.2%). Malaria parasite and sickling status proved negative. Alanine transaminase (ALT) and aspartate transaminase (AST) were marginally raised and the total protein was slightly low although serum albumin was markedly reduced (22.0 g/dl). Chest X-ray showed minute pneumoperitoneum below the right diaphragm (Figure 2).
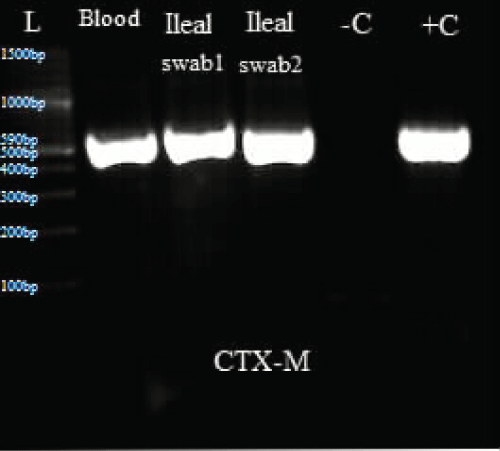
.
Figure 3: Represents PCR results for CTX-M.
L: Ladder; +C: Positive controls; -C: Negative controls; Blood: Isolate from blood sample; Ileal swab 1: Isolate from perforated site; Ileal swab 2: Isolate from sutured site.
View Figure 3
Identification Procedure of Microorganisms and Antimicrobial Tests
Blood culture
The blood culture sample was incubated in Bactec 9050 at 37 °C. The blood culture flagged positive after 24 hours of incubation and was also sub-cultured on blood and Mackonkey agar media. The Growth characteristics showed pure lactose fermentation on Mackonkey agar and non-haemolytic colonies on blood agar. The colonies were inoculated in Citrate agar (BD, France), Kliegler iron agar (BD, France) and Urea agar and biochemical characteristics showed features consistent with E.coli. The isolate was subjected to analytical profile index 20E (Biomerieux, France) testing and a profile of 5144572 was generated giving an identification of Escherichia coli..
Antimicrobial susceptibility testing was carried out using the Kirby-Bauer disk diffusion method following the Clinical Laboratory Standards Institute guidelines (CLSI) [10]. The antimicrobials used were ampicillin (10 μg), chloramphenicol (30 μg), co-trimoxazole (25 μg), ciprofloxacin (5 μg), amoxicillin (25 μg), ceftazidime (30 μg), gentamicin (10 μg), cefuroxime (30 μg) and tetracyclin (30 μg). The isolates were resistant to all antimicrobials. The isolate was subjected to a second line testing using meropenem (10 μg) and this time they were susceptible. Based on the resistance pattern of ceftriazone and ceftazidime, confirmatory tests for ESBL production were performed using the double disc synergy method as described in the CLSI guidelines [10]. Briefly, 0.5 McFarland suspensions of the isolates were inoculated in Mueller Hinton agar. Antibiotic disc of ceftazidime (30 μg) and cefotaxime (30 μg) were placed beside ceftazidime/clavulanate (30/10 μg), and cefotaxime/clavulanate (30/10 μg) respectively. After incubation, inhibition zone diameters were measured and interpreted. A zone difference of ≥ 5 mm was observed between the single (cefotaxime and ceftazidime discs) and corresponding combination disks (cefotaxime/clavulanate and ceftazidime/clavulanate) and hence noted as ESBL positive. The ATCC strains used for antimicrobial susceptibility testing and phenotypic confirmation of ESBL were E.coli ATCC 25922 and Klebsiellapneumoniae ATCC.
Swab cultures
The two swab samples taken from the perforated site were also sub-cultured on blood agar and Mackonkey agar (BD, France) and incubated at 37 °C overnight. Direct examination of the swab samples yielded Gram negative rods. After overnight incubation, pure lactose fermenting bacteria were isolated and further biochemical and API 20E investigations identified the bacteria as E.coli. The antimicrobial susceptibility test yielded the same pattern as those of the blood isolate and phenotypic typing for ESBL showed positive.
Stool cultures follow-up
Follow up stool samples collected after patient has been discharged was sub-cultured directly on Mackonkey agar and Xylose lysine deoxycholate agar (XLD). Analytical profile index of the isolate yielded E.coli with similar colony morphology as previous isolates. The isolate was however susceptible to all antimicrobials and phenotypic testing for ESBL was negative.
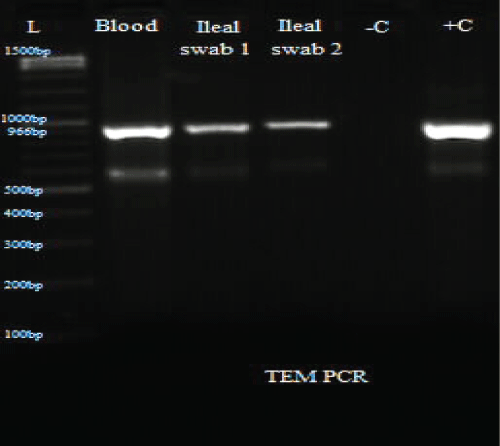
.
Figure 4: PCR results for TEM.
L: Ladder; +C: Positive controls; -C: Negative controls; Blood: Isolate from blood sample; Ileal swab 1: Isolate from perforated site; Ileal swab 2: Isolate from sutured site.
View Figure 4
ESBL typing of E.coli strains
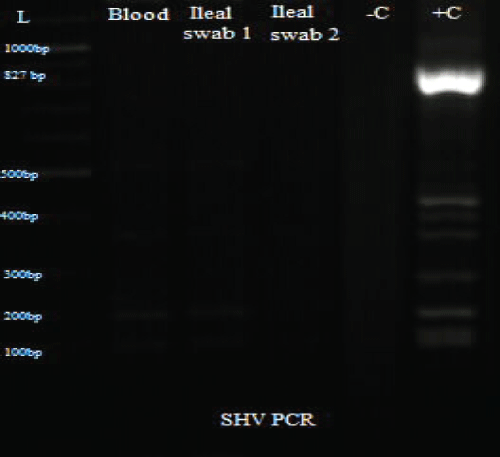
Total DNA was extracted from the swab and blood isolates using Spherolyse DNA extraction kit (Hain Life science GmbH, Germany), following the manufacturer's instructions. The extracts were subjected to single plex conventional PCR analysis targeting the blaSHV-, blaTEM- and blaCTX-M-encoding genes. Fifty(50- μl) reaction mixes consisted of 5 μl of 10X reaction buffer, 4 μl of 25 mM MgCl2, 1 μl of 10 mM dNTPs, 1 μl of forward primer, 1 μl of reverse primer, 0.25 μl of 5 units Taq DNA polymerase and 5 μl of DNA template. The final concentration of each primer was 0.2 μM. Amplifications were carried out with the following thermal cycling profile: initial denaturation for 3 mins at 94 °C, followed by 35 cycles of amplification consisting of 94 μC for 45 s, annealing at 56 μC for 35 s and primer extension at 72 μC for 30 s. A final extension cycle at 72 μC for 10 mins with a holding step at 4 μC was included to finish the entire PCR amplification. In the case of CTX-M, an annealing temperature of 62 μC for 45 s was used. Primers sequences are shown in table 1. PCR results showed the three bacterial isolates were positive for TEM and CTX-M (Figure 3, Figure 4 and Figure 5).
.
Figure 5: PCR results for SHV.
L: Ladder; +C: Positive controls; -C: Negative controls; Blood: Isolate from blood sample; Ileal swab 1: Isolate from perforated site; Ileal swab 2: Isolate from sutured site.
View Figure 5
![]()
Table 1: List of primers and their product size.
View Table 1
Discussion
ESBL-producing organisms pose a serious therapeutic challenge because of their ability to hydrolyse B-lactams and other associated antibiotics including quinolones [11,12]. Although some studies have demonstrated the presence of ESBL-mediated resistance in bacteria causing infections in patients in Ghana and other developing countries, few reports have shown the real impact of this bacterium in patients with ileal perforation.
Both molecular and phenotypic investigations confirmed the blood and swab isolates as being β-lactamase-producing E.coli harbouring ablaCTX-M gene and ablaTEM gene. Reports on the association between ESBL-producing E.coli and ileal perforations are quite rare and perhaps under-reported in developing countries because of the general lack of laboratory capacity for identifying the causative agents involved.
Most laboratory diagnosis of typhoid infections in developing countries are based on Widal test other than culture confirmed blood or peritoneal fluid. Widal test, however, has been found to be non-specific and difficult to interpret in areas where typhoid fever is endemic [13]. Majority of enteric perforations diagnosed as due to Salmonella organisms could therefore be only based on conjecture. Our report therefore shows a classical example of an E.coli-associated ileal perforation occurring about 30 cm away from the ileocaecal junction along the antimesenteric border. This pattern of perforation is similar to the reported cases of typhoid-associated ileal perforations [14,15].
A similar report by Capoor, et al. [16] identified E.coli (23.4%) as being the most predominant isolate out of 47 ileal perforations studied in Northern India. In the same report, only 10.5% of the ileal perforations were due to enteric fever. Similar findings were reported by previous studies [17,18].
One important point of note is the resistant pattern exhibited by the isolated bacteria and its likely impact on patient management. In Ghana and many developing countries, the clinical algorithm for managing patients with ileal perforations is to perform early surgery and administer blind therapy, mostly broad-spectrum antibiotics such as cephalosporins and quinolones. This algorithm is based on the fact that most ileal perforations are due to Salmonella, which is generally susceptible to these drugs. The isolation of an ESBL-producing E.coli is therefore quite significant to public health and attending physicians and calls for the need for laboratory capacity to be improved in at least strategic laboratories working closely with the Ghana Health Service in order to increase diagnosis of these infections. Carbapenems, which are the recommended choice for managing ESBL-producing bacteria, are quite expensive in Ghana and many developing countries. A full 7-day regimen costs around $1,200. Even the most urban dwellers cannot afford this drug, least of all rural peasant farmers as is the case of this patient. Similar cases of invasive ESBL-producing which are unrelated to ileal perforations, have been reported in the cerebrospinal fluid, ear discharges and blood samples [19,20].
One question we could not answer was the type of infection the patient had, whether community- or hospital-acquired. This is because we only got opportunity of taking samples for cultures after the patient had spent 10 days at the health centre where she was referred. Literature, however, shows hospitalisation is not a risk factor for intestinal colonisation with blaCTX-M type producing E.coli, thus possibly suggesting that our patient might have community-acquired ESBL-producing E.coli [21].
Although we are also unable to tell the source of this particular bacterium, we do not think it has any relationship with faecal carriage or possible enteric colonisation since the stool sample collected after discharge was negative for ESBL E.coli.
Conclusion
Our report highlights a possible association between ESBL producing E.coli and ileal perforation. Physicians are encouraged to rely on evidence through appropriate antibiotic susceptibility testing before resorting to the use of antimicrobial agents.
Ethical Approval and Consent to Participate
We obtained consent from the parents of the child before proceeding with publication of this report.
Consent for Publication
Written informed consent was obtained from the parents of the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of Data and Material
All data generated or analysed during this study are included in this published article.
Competing Interests
The authors declare no competing interests. No author has any proprietary interest in any of the products or ideas mentioned in this article.
Funding
The material and reagents for these investigations were supported by the EOD research group - KCCR.
Acknowledgements
We thank the parents of the patient for giving us consent to publish this manuscript. We also express our appreciation to Godfred Acheampong and Abednego Arthur for supporting us with laboratory testing of the specimen.
Authors' Contributions
KTM, NS, JA and IO reviewed the patient, and contributed to writing the manuscript. KSM, AA and MO performed laboratory analysis of the samples. EOD, YAS and MO documented the clinical findings, validated clinical and laboratory data and contributed to writing the manuscript. All authors have read and approved the final manuscript.
References
-
Coyte A, Tahar Aissa J, Koh HC, Mackay G (2016) Case of unrecognised food bone ingestion with dual site intestinal perforations. BMJ Case Rep 2016.
-
Imataki O, Shiroshita K, Uchida S, Kida J, Akamoto S, et al. (2016) Perforation in an intestinal malignant lymphoma case. BMC Res Notes 9: 308.
-
Moreno-Aguilera E, Galeana-Nogueda FI, Vera-Aguilera J, Vera-Aguilera C, Ley-Marcial LA (2016) Jejunal perforation secondary to pulmonary mucoepidermoid carcinoma metastasis. Case report and review.
-
Naselli A, Garaventa A, Buffa P, Granata C, Bandettini R, et al. (2016) Primary intestinal mold infection in children with solid tumors: a case report in an adolescent with Ewing sarcoma, and literature review. New Microbiol 39: 232-234.
-
Agarwal S, Gera N (1996) Tuberculosis--an underestimated cause of ileal perforation. J Indian Med Assoc 94: 341-352.
-
Birgy A, Levy C, Bidet P, Thollot F, Derkx V, et al. (2016) ESBL-producing Escherichia coli ST131 versus non-ST131: evolution and risk factors of carriage among French children in the community between 2010 and 2015. J Antimicrob Chemother 71: 2949-2956.
-
Fischer J, Hille K, Ruddat I, Mellmann A, Kock R, et al. (2016) Simultaneous occurrence of MRSA and ESBL-producing Enterobacteriaceae on pig farms and in nasal and stool samples from farmers. Vet Microbiol.
-
Ljungquist O, Ljungquist D, Myrenas M, Ryden C, Finn M, et al. (2016) Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs - a pilot study. Infect Ecol Epidemiol 6: 31514.
-
Palma N, Gomes C, Riveros M, Garcia W, Martinez-Puchol S, et al. (2016) Virulence factors profiles and ESBL production in Escherichia coli causing bacteremia in Peruvian children. Diagn Microbiol Infect Dis 86: 70-75.
-
Clinical and Laboratory Standard Institute (2014) M100-S24, Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. In Book M100-S24, Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Fourth Informational Supplement 34.
-
Shaikh S, Fatima J, Shakil S, Danish Rizvi SM, Kamal MA (2015) Prevalence of multidrug resistant and extended spectrum beta-lactamase producing Pseudomonas aeruginosa in a tertiary care hospital. Saudi J Biol Sci 22: 62-64.
-
Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA (2015) Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci 22: 90-101.
-
Chaudhry R, Shankar S, Nisar N, Dey AB (1995) Recent advances in the diagnosis of enteric fever. Tropical gastroenterology : official journal of the Digestive Diseases Foundation 16: 8-12.
-
Abdul Ghaffar Ansari, Syed Qaiser Hussain Naqvi, Ali Akbar Ghumro, Abdul Hakeem Jamali, Talpur AA (2009) Management of Typhoid Ileal Perforation: A Surgical Experience of 44 Cases. Gomal Journal of Medical Sciences.
-
Na'aya HU, Eni UE, Chama CM (2004) Typhoid Perforation in Maiduguri, Nigeria. Annals of African Medicine 3: 69-72.
-
Capoor MR, Nair D, Chintamani MS, Khanna J, Aggarwal P, et al. (2008) Role of enteric fever in ileal perforations: an overstated problem in tropics? Indian J Med Microbiol 26: 54-57.
-
Bhansali SK (1967) Gastrointestinal perforations. A clinical study of 96 cases. J Postgrad Med 13: 1-12.
-
Brook I (2003) Microbiology and management of intra-abdominal infections in children. Pediatr Int 45: 123-129.
-
Elaldi N, Gozel MG, Kolayli F, Engin A, Celik C, et al. (2013) Community-acquired CTX-M-15-type ESBL-producing Escherichia coli meningitis: a case report and literature review. J Infect Dev Ctries 7: 424-431.
-
Oduro-Mensah D, Obeng-Nkrumah N, Bonney EY, Oduro-Mensah E, Twum-Danso K, et al. (2016) Genetic characterization of TEM-type ESBL-associated antibacterial resistance in Enterobacteriaceae in a tertiary hospital in Ghana. Ann Clin Microbiol Antimicrob 15:29.
-
Rossolini GM, D'Andrea MM, Mugnaioli C (2008) The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect 1: 33-41.
-
Chanawong A, M'Zali FH, Heritage J, Lulitanond A, Hawkey PM (2000) Characterisation of extended-spectrum beta-lactamases of the SHV family using a combination of PCR-single strand conformational polymorphism (PCR-SSCP) and PCR-restriction fragment length polymorphism (PCR-RFLP). FEMS Microbiol Lett 184: 85-89.
-
Arpin C, Dubois V, Coulange L, Andre C, Fischer I, et al. (2003) Extended-spectrum beta-lactamase-producing Enterobacteriaceae in community and private health care centers. Antimicrob Agents Chemother 47: 3506-3514.
-
Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L (2003) Prevalence and Molecular Epidemiology of CTX-M Extended-Spectrum ß-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in Russian Hospitals. Antimicrob Agents Chemother 47: 3724-3732.





