Clinical Medical
Reviews and Case Reports
Cytotoxic Effect of Snake (Echis Carinatus) Venom on Human Embryonic Kidney Cells (HEK 293)
Mahboobeh Balali Bahadorani1 and Abbas Zare Mirakabadi2*
1Department of Biology, Karaj Branch, Islamic Azad University, Iran
2Department of Venomous Animals and Antivenom Production, Razi Vaccine and Serum Research Institute, Iran
*Corresponding author: Abbas Zare Mirakabadi, Professor, Biochemistry, Department of Venomous Animals and Antivenom Production, Razi Vaccine and Serum Research Institute, Karaj, Iran, Tel: 02634552006, E-mail: zareabbas83@gmail.com
Clin Med Rev Case Rep, CMRCR-3-132, (Volume 3, Issue 9), Original Article; ISSN: 2378-3656
Received: July 12, 2016 | Accepted: September 23, 2016 | Published: September 26, 2016
Citation: Bahadorani MB, Mirakabadi AZ (2016) Cytotoxic Effect of Snake (Echis Carinatus) Venom on Human Embryonic Kidney Cells (HEK 293). Clin Med Rev Case Rep 3:132. 10.23937/2378-3656/1410132
Copyright: © 2016 Bahadorani MB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The venom of snake (Echis carinatus) induces hemorrhage and necrosis locally at the bite site as well as acute renal failure (ARF) as a consequence of morphological and functional alterations in glomerular and tubular cells.
Objectives: It is not clear that ARF results from a direct cytotoxic effect on renal epithelia or from a renal ischemia due to systemic hemodynamic disturbances. This work investigated the in vitro effect of Echis Carinatus crude venom, using cultured Human embryonic kidney (HEK 293) mono layers as a model to see the cytotoxic effect of Echis carinatus venom.
Materials and methods: The effect of Echis Carinatus snake venom on HEK 293 cells viability was determined by MTT assay and neutral red uptake assay. The integrity of cell membrane through LDH release was measured with the LDH Kit. Morphological changes of endothelial cells were also evaluated using a phase contrast microscope.
Results: In MTT assay, crude venom induced dose dependent cytotoxic effects on HEK 293 cells which were confirmed by neutral red assay. Crude venom caused changes in the integrity of cell membrane determined by rise in LDH release too.
Conclusions: Based on the results obtained in the present study it may be concluded that the damage induced by E. carinatus venom on Kidney is probably related to the direct effect as well as indirect effect including hypotension, hemolysis, hemoglobinuria, rhabdomyolysis, and myoglobinuria of this venom on kidney which may lead to Acute renal failure (ARF).
Keywords
Snake venom, Cytotoxicity effect, HEK 293, Acute renal failure, Echis carinatus
Introduction
The morbidity and mortality associated with snake bite are serious public health problems in many regions of the world [1]. It is estimated that the true incidence of snake envenomation could exceed 5 million per year [2,3]. Viper bites are more common than other poisonous snake ¬bites in human beings. Echis carinatus snakes due to presence of specific enzymes like metalloproteases (SVMPs), hyaluronidases and phospholipases A2 (PLA2s), which often complement each other's functions, make progressive tissue necrosis and permanent physical deformities [4,5]. It seems that metalloproteases can cause lysis of structural proteins including basal lamina [6]. It is well established fact that some snake venoms including E. carinaus can cause local tissue damage which brings about pain and edema leading to performance tissue loss. On the other hand the venom can cause systemic effects including, anemia, hypotension hemorrhage as well as acute renal failure [7,8]. Acute renal failure (ARF) is mainly observed following bites by the snakes which belong to the viperidae group [9,10]. The ARF which happen after snake bite is usually reversible, but if acute cortical necrosis occurs, it may lead to an incomplete recovery [11]. Acute Kidney Injury is diagnosed by biochemical monitoring which presents a late indication of a functional change in glomerular function rate [12]. It is thought that Echis carinatus snake venom can induces ARF as a consequence of morphological and functional alterations in glomerular and tubular cells [13]. A few reports indicate the cytotoxicity of Echis venom [14,15]. Hoda Khalid (2015) recently investigated the cytotoxic effect of crude venom of Echis ocellatus [14]. Moreover the Rebecca D Pierce (2011) used polyethyleneimine (PEI) to enhance cellular adherence, and to determine whether the substrate attachment influenced the survival of cells treated with crude E. carinatus venom. It is still unclear whether the ARF results from a direct cytotoxic effect on renal epithelia or from a renal ischemia due to systemic hemodynamic disturbances [15]. In order to elucidate a putative direct cytotoxic action of Echis carinatus venom we used an in vitro system, using cultured Human embryonic kidney (HEK 293) cells monolayers as a model.
Materials and Methods
Venom preparation
Ten milligrams of lyophilized Echis carinatus crude venom was obtained from the Venomous Animals and Antivenom Production Department of Razi Vaccine and Serum Research Institute, Iran. The venom was dissolved in 10 ml of DMEM culture media and stored at -20°C until use.
Reagents
Dulbecco's Modified Eagle's medium, high glucose ( DMEM), Fetal Bovine Serum (FBS), Penicillin-Streptomycin were purchased from Gibco BRL (Life Technologies, Paisley, Scotland), MTT(3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), neutral red dye (NR), DMSO (dimethyl sulfoxide) were obtained from Merck (Germany), LDH (Lactate Dehydrogenase) assay Kit were purchased from Pars azmoon, Iran, Human embryonic kidney (HEK 293) cells obtained from the Venomous Animals and Antivenom Production Department of Razi Vaccine and Serum Research Institute, Iran.
Cell culture
Normal human embryonic kidney cells (HEK 293), grown in plastic flasks at 37°C in humidified atmosphere of 5% CO2/air with DMEM supplemented with 10% Fetal Bovine Serum FBS and 1% penicillin (10,000 IU/ml)/streptomycin (50 mg/ml).
Determination of cell viability (MTT assay)
HEK 293 cells were cultured in DMEM medium in the presence of FBS 10% plus penicillin-streptomycin 1%, and incubated in presence of CO2 5% at 37°C. The cytotoxicity of Echis Carinatus crude venom was evaluated using MTT assay. HEK 293 cells were seeded in a 96 well plate at 3-4 × 104 cells/well and incubated for 24 hr to adhere. After discarding the old medium, the cells were exposed in the medium containing various concentrations 1, 5, 10, 20, 40, 80 μg/mL of crude venom. After 3 and 24 hr exposure, 20 μL MTT (5 mg/mL) was added to each well and cells were incubated for another 4 hr. Finally, the culture medium containing MTT solution was removed and the Formazan crystals were dissolved in 150 μL of dimethyl sulfoxide solvent (DMSO). Absorbance was read at 540 nm with a microplate reader (Labsystem Multiskan MS 4.0, Finland). IC50 was calculated using the Sigma Plot 12.0 software.
Neutral red uptake assay
For the neutral red (NR) cytotoxicity assay, the HEK 293 cells were seeded in 96-well plates at a cell density of 3-4 × 104 cells/well. Following venom exposure (as mentioned above in MTT assay), the media were removed and the culture was washed once with phosphate buffered saline (PBS), pH 7.4. To each well 100 μl of media containing NR (40 μg/mL) was added and the plate was incubated for 3 hr at 37°C. The media containing the dye were removed and each well was washed once for 2-3 min with formol-calcium (40% formaldehyde, 10% anhydrous calcium chloride, w/w) to remove non incorporated dye. Finally, 200 μl of an acetic acid-ethanol (1 ml glacial acetic acid in 100 ml 50% ethanol) was added to each well for 15 min at room temperature and then the plate was read at 540 nm in a microplate reader. The cell viability was determined by comparing the absorbance values of all the wells with the absorbance mean value obtained from the control wells (without venom), which were taken as 100% cell viability.
Lactate dehydrogenase (LDH) release assay
In order to quantify the cell death, lactate dehydrogenase (LDH) released from damaged cells into the cell culture media was measured 3 and 24 hr after treatment by the E. carinatus crude venom with various concentrations. Cells were seeded in 96-well plate at a density of 3 × 104 cells/well in culture medium. After overnight incubation, the medium was replaced with serum free medium containing various concentrations of E. carinatus crude venom and incubated for 3 and 24 hr. A colorimetric assay was applied according to (Pars azmoon, Iran) kit, and then the content of LDH released from the cells to the culture medium was calculated according to recipe kit.
Morphological studies
Following overnight incubation of the cells with venom, various morphological alterations and cell damage were qualitatively investigated using a invert microscope and their photos were taken with a digital camera.
Statistical analysis
Experiments were performed in triplicate with four replicates for each exposure concentration and results are expressed as mean ± SD. Data were analyzed by Student t-test and an analysis IC50 by fitting the data to log (inhibitor) vs. response equation. Significance level of p < 0.05 was used for statistical testing. All statistical analyses were performed using Sigma Plot 12 software.
Results
Determination of cell viability (MTT assay)
The inhibitory effects of crude venom of E. carinatus on growth inhibition of HEK 293 cells were tested at various concentrations (1 to 80 μg/ml) for 3 and 24 hr using colorimetric MTT assay. Data analysis showed (Figure 1) that the growth inhibition of HEK 293 cells exposed to the venom were increased significantly (p < 0.01) as compared to venom unexposed cells in concentration-dependent manner. The maximum cell inhibition was 69% after 3 hr and 75% after 24 hr in 80 μg/ml concentration exposure respectively and the least cell inhibition was 19% after 3 hr and 30% after 24 hr in 1 μg/ml concentration respectively. The IC50 (half maximal inhibitory concentration) value of E. carinatus snake venom on HEK 293 cell was 18.54 ± 8.96 μg/mL and 14.06 ± 3.17 μg/mL after 3 and 24 hours exposure respectively.
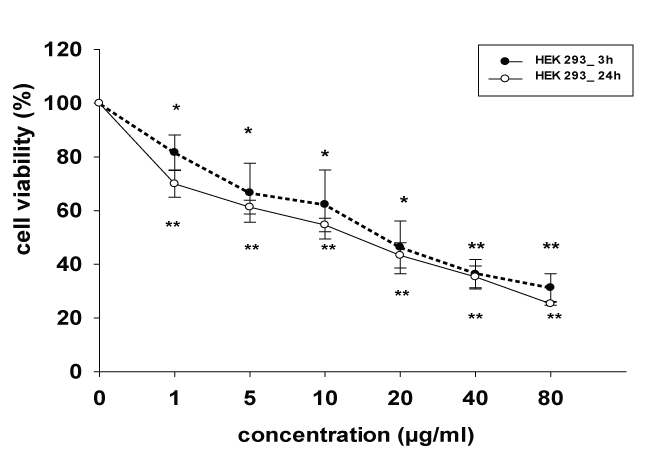
.
Figure 1: Cytotoxic effect of E.carinatus crude venom on HEK 293 cell line, cell viability after exposure to various concentrations of venom for 3 and 24 hr. Cell viability was determined using by MTT assay. The control value (without venom) was set as 100%. Data are expressed as the mean ± SD. *p < 0.05 and **p < 0.01 were considered to be statistically significant in comparison with control.
View Figure 1
Neutral red uptake assay
Cell viability and effects of cytotoxicity on lysosomal integrity was determined with the neutral red (NR) assay. As seen in figure 2. Following venom exposure cells lysosomal neutral red uptake reduced with an IC50 value of 16.66 ± 1.26 μg/mL and 8.43 ± 0.54 μg/mL after 3 and 24 hours incubation respectively.
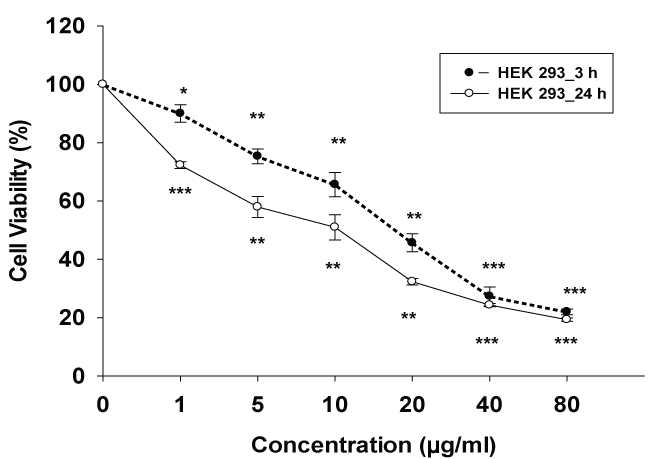
.
Figure 2: Cytotoxic effect of E.carinatus crude venom on HEK 293 cell line, cell viability after exposure to various concentrations of venom for 3 and 24 hr. Cell viability was determined using by neutral red uptake assay (NR). The control value (without venom) was set as 100%, data are expressed as the mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered to be statistically significant, compared with controls.
View Figure 2
Data analysis showed that the cell inhibition of HEK 293 cells exposed to the venom was significantly (p < 0.001) inhibited as compared to control cells in concentration dependent manner. The maximum cell inhibition was 78% after 3 hr and 81% after 24 hr in 80 μg/ml concentration exposure with E. carinatus snake venom and the least cell inhibition was 10% after 3 hr and 28% after 24 hr in 1 μg/ml concentration exposure.
Lactate dehydrogenase (LDH) release assay
Figure 3 shows the values of LDH released from the HEK 293 cell line after 3 and 24 hours of incubation with E. carinatus venom at concentrations ranging from 1 to 80 μg/ml. The effect of venom on LDH release was concentration dependent. Treatment of HEK 293 cells with E. carinatus snake venom at concentrations 1, 5, 10 and 20 μg/ml for 3 hrs caused LDH release by about 1.5 to 2 folds as compared to control but statistical analysis did not show significant. However when the concentration increased to 40 μg/ml and above, LDH activity in the cultured media increased significantly (p < 0.01). At maximum concentration (80 μg/ml) the rise in LDH activity was by 3 folds as compared with unexposed cells after 24 hr exposure.
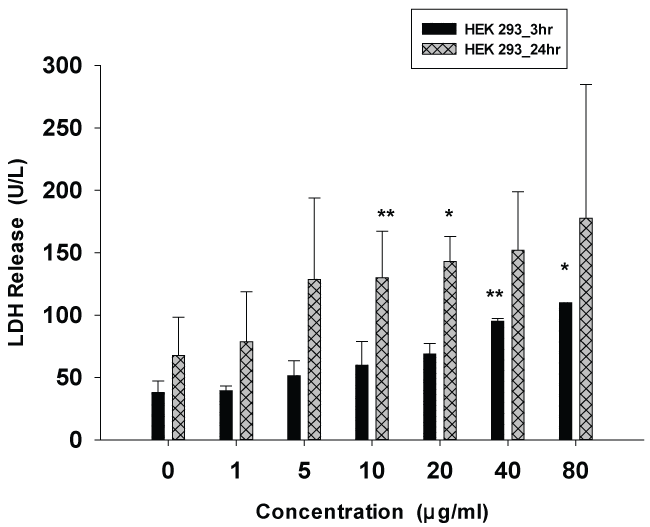
.
Figure 3: Effect of E.carinatus crude venom on HEK 293 cells, growth inhibition determined by LDH release assay. Values indicate mean ± SD of LDH activity (U/L) (compare to control) for 3 and 24 hr. *p < 0.05 and **p < 0.01 were considered to be statistically significant, compared with values from cells incubated in the absence of E.carinatus crude venom (controls).
View Figure 3
Morphological studies
Untreated HEK 293 cells were homogeneously distributed in the culture field exhibiting a polygonal shape with distinct boundaries. Various morphological abnormalities was observed in cells exposed to various concentration and showed that HEK 293 cells lost their common polygonal shape and appeared in a form of numerous roughly rounded cells of variable size. Areas devoid of cells were also recorded. The treatment with 10 μg/ml to 80 μg/ml of venom led to the aggregation of dense irregular cellular debris. No intact cells were recognized in this medium, which indicates the occurrence of widespread cell death. Interestingly, the morphological changes that showed after 3hr incubation was similar to 24 hr incubation (Figure 4).
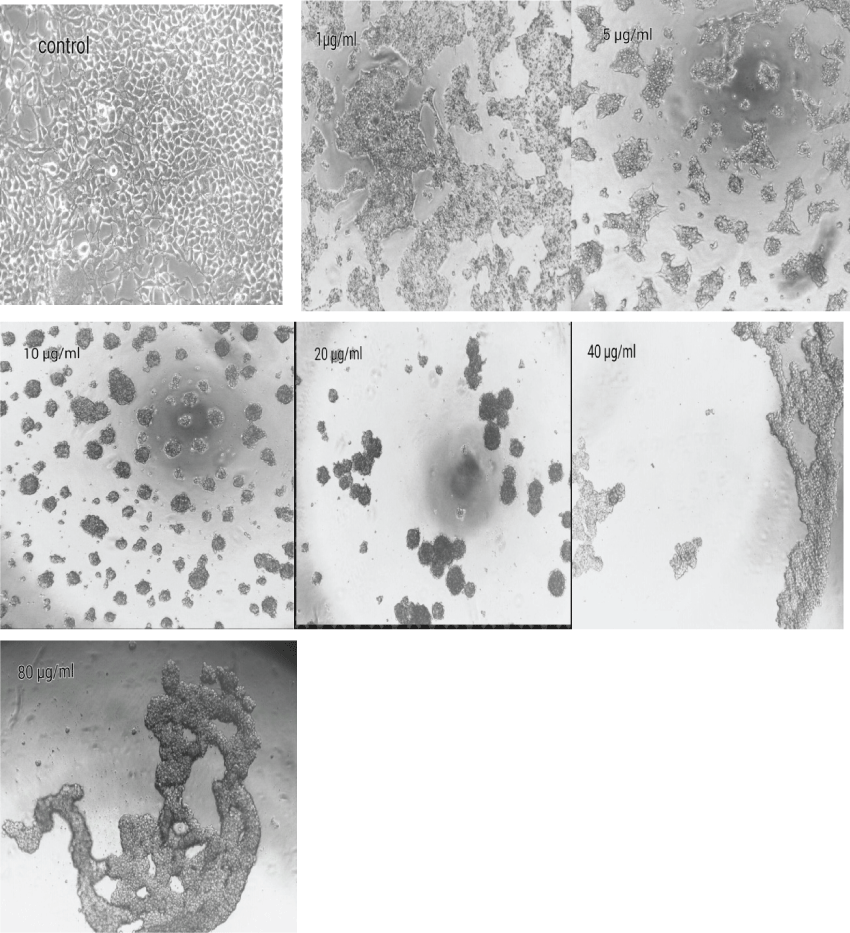
.
Figure 4: Effect of E.Carinatus crude venom on morphology of HEK 293 cells. Cells were seeded in DMEM medium with 10% FBS and treated in the absence (control group) or crude venom concentrations for 3 and 24 hr at 37°C. The Morphological changes of treated cells that showed after 3 hours exposure were similar to 24 hours exposure. Inverted microscope was used to compare with control cells in this study.
View Figure 4
Discussion
Echis carinatus venom is a highly complex mixture of a variety of biological substances including protein and non protein toxins which degrade tissue structure and promote hemorrhaging [16]. In this study, we have demonstrated a direct cytotoxic effect of Echis carinatus crude venom by exposing the HEK 293 cells to various concentrations of crude venom for 3 and 24 hour using MTT, Neutral red and LDH assay. The cell line HEK 293 was used in this study. Most cells derived from an embryonic kidney would be endothelial, epithelial, or fibroblasts. Although HEK 293 is not clearly represent kidney tissue, but since the origin of the cells is human embryonic Kidney, it can play a role as a related model to kidney tissue. The primary effects of crude venom of Echis carinatus on HEK 293 cell was induction of changes in the cell shape and detachment of cell from the surface of plate and subsequent aggregation which examined by phase-contrast microscopy. The detachment of cells may be due to the disintegrin in the venom of E. carinatus [17-19]. Disintegrins are non enzymatic proteins which binds to integrin receptors T resulting in competitive inhibition of integrin binding to extracellular matrix proteins [15,19,20]. The results in present study are in accordance with the results obtained by Hoda Khalid (2015) that recently reported the cytotoxicity effect of crude venom of Echis on rat skeletal muscle cell line (L6) and evaluated the concentration-dependent inhibition of cells exposed to the venom [14]. Recently cytotoxic effect of V. lebetina crude venom on the HUVEC, Bothrops moojeni crude Venom on MDCK and Russell's viper venom on human A549 cells were reported by various research workers [21-23]. Also, Michael Conlon (2013) investigated the cytotoxic activities of purified phospholipase A2 (Ser49) from the venom of the Echis carinatus on lung adenocarcinoma A549 cells and human umbilical vein endothelial cells (HUVECs) and showed concentration- dependent inhibition of cells [24]. Some in vivo studies recently reported the effect of Echis carinatus venom on kidney and showed the necrotic effect of this venom to cause acute renal failure [25]. We used two colorimetric assay MTT and Neutral red to determine the cytotoxicity of the venom [26,27]. The results of NR and MTT assays are often comparable [28]. Vian, et al. (1995) reported close correlations between the NR and MTT assays for most test chemicals [29,30]. In our study, the MTT and neutral red (NR) assay showed that Echis carinatus venom has cytotoxic effect on HEK 293 cells in a concentration-dependent manner after 3 and 24 hours exposure. The results obtained from MTT assay after exposure of cells to the crude venom for 3 hours is more or less similar to the results obtained after exposing for 24 hours. This may be due to the necrotic effect of the venom on cells rather than apoptotic nature of the venom. Susan Elmore (2007) reported that the apoptosis is a time-consuming process; hence the results obtained in the present study may reveal the necrotic effect of the venom rather than apoptotic effect [31].
The cell line HEK 293 was used for cytotoxicity assay of the venom on kidney cells [32] In order to further characterize crude E. carinatus venom cytotoxicity, we examined plasma membrane integrity. Because SVMP have been shown to induce plasma mem¬brane disintegrity [33]. We used the LDH assay an ubiquitous cytosolic enzyme which releases if the plasma membrane of cells injured [34,35]. The results obtained indicate that the effect of E. carinatus venom on cells was dose related. This effect was significant when compared to the control values at 3 hours. However the rise in activity of LDH at 24 hours exposure was non-significant. This can be due to high standard deviation (SD) in the results obtained at 24 hours exposure. The release of LDH into the culture medium and results of cell viability that obtained after 3 and 24 hours exposure with MTT and NR assay gives an accurate measure of cellular toxicity induced by to the venom and correlates well with the severity of cell death and membrane damage observed in this study.
Conclusion
Based on the results obtained in the present study it may be concluded that the damage induced by E. carinatus venom on Kidney is probably related to the direct effect as well as indirect effect including hypotension, hemolysis, hemoglobinuria, rhabdomyolysis, and myoglobinuria of this venom on kidney which may lead to Acute renal failure (ARF).
Acknowledgments
The authors thank Razi Vaccine and Serum Research Institute, Karaj, Iran for providing us the lyophilized crude Echis carinatus venom and other materials for our research.
References
-
Al Qahtani MA, Altheaby A, Al Anazi T, Al Saad K, Binsalih S, et al. (2014) Snake bite complicated by acute kidney injury secondary to necrotizing glomerulonephritis. Saudi J Kidney Dis Transpl 25: 1259-1262.
-
Hasson SS, Al Jabri AA, Sallam TA, Al Balushi MS, Mothana RA (2010) Antisnake Venom Activity of Hibiscus aethiopicus L. against Echis ocellatus and Naja n. nigricollis. J Toxicol 2010: 837864.
-
Monteiro FN, Kanchan T, Bhagavath P, Kumar GP, Menezes RG, et al. (2012) Clinico-epidemiological features of viper bite envenomation: a study from Manipal, South India. Singapore Med J 53: 203-207.
-
Simon C Wagstaff, Robert A Harrison (2006) Venom gland EST analysis of the saw-scaled viper, Echis ocellatus, reveals novel a9ß1 integrin-binding motifs in venom metalloproteinases and a new group of putative toxins, renin-like aspartic proteases. Gene 377: 21-32.
-
Nanjaraj Urs AN, Yariswamy, Vikram Joshi M, Suvilesh KN, Sumanth MS, et al. (2015) Local and systemic toxicity of Echis carinatus venom: neutralization by Cassia auriculata L. leaf methanol extract. J Nat Med 69: 111-122.
-
Savanur A, Ali SA, Munir I, Abbasi A, Alam M, et al. (2014) Pharmacological and biochemical studies on the venom of a clinically important viper snake (Echis carinatus) of Pakistan. Toxicon 80: 47-57.
-
Ramos OH, Selistre-de-Araujo HS (2006) Snake venom metalloproteases--structure and function of catalytic and disintegrin domains. Comp Biochem Physiol C Toxicol Pharmacol 142: 328-346.
-
Kularatnea SAM , Sivansuthanb S, Medagedaraa SC, Maduwagec K, de Silva A (2011) Revisiting saw-scaled viper (Echis carinatus) bites in the Jaffna Peninsula of Sri Lanka: distribution, epidemiology and clinical Manifestations. Trans R Soc Trop Med Hyg 105: 591-597.
-
Basu J, Majumdar G, Dutta A, Sengupta SK, Kundu B, et al. (1977) Acute renal failure following snake bite (viper). J Assoc Physicians India 25: 883-890.
-
Chugh KS (1989) Snake-bite-induced acute renal failure in India. Kidney Int 35: 891-907.
-
L H, A JL, H LT, B RH, Metri SS (2013) A study on the acute kidney injury in snake bite victims in a tertiary care centre. J Clin Diagn Res 7: 853-856.
-
Thamarai R, Sivakumar K (2014) Plasma netrophil gelatinase associated lipocalin as an early biomarker of acute kidney injury in snake bite. J of Evolution of Med and Dent Sci 3:13537-13546.
-
Oram S, Ross G, Pell L, Winteler J (1963) Renal cortical calcification after snake-bite. Br Med J 1: 1647-1648.
-
Khalid H, Mohammed Mukhtar M, Konstantakopoulos N (2015) Cytotoxiciy of Naja nubiae (Serpentes: Elapidae) and Echis ocellatus (Serpentes: Viperidae) Venoms from Sudan. J Toxicol 2015: 7.
-
Pierce RD, Kima ES, Girton LW, McMurry JL, Francis JW, et al. (2011) Characterization of crude Echis carinatus venom-induced cytotoxicity in HEK 293T cells. J Venom Res 2: 59-67.
-
Fonseka CL, Jeevagan V, Gnanathasan CA (2013) Life threatening intracerebral haemorrhage following saw- scaled viper (Echis carinatus) envenoming-authenticated case report from Sri Lanka. BMC Emerg Med 13: 5.
-
Marcinkiewicz C , Calvete JJ, Marcinkiewicz MM, Raida M, Vijay-Kumar S, et al. (1999) EC3, a Novel Heterodimeric Disintegrin from Echis carinatus Venom, Inhibits a4 and a5 Integrins in an RGD-independent Manner. J Biol Chem 274: 12468-12473.
-
Escalante T, Shannon J, Moura-da-Silva AM, Gutiérrez JM, Fox JW (2006) Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Arch Biochem Biophys 455: 144-153.
-
Kamiguti AS, Zuzel M, Theakston RD (1998) Snake venom metalloproteinases and disintegrins: interactions with cells. Braz J Med Biol Res 31: 853-862.
-
Yamada D, Sekiya F, Takashi Morita (1996) Isolation and Characterization of Carinactivase, a Novel Prothrombin Activator in Echis carinatus Venom with a Unique Catalytic Mechanism. J Biol Chem 271: 5200-5207.
-
Kakanj M, Ghazi-Khansari M, Zare Mirakabadi A, Daraei B, Vatanpour H (2015) Cytotoxic Effect of Iranian Vipera lebetina Snake Venom on HUVEC Cells. Iran J Pharm Res 14: 109-114.
-
Collares-Buzato CB, de Paula Le Sueur L, da Cruz-Höfling MA (2002) Impairment of the Cell-to-Matrix Adhesion and Cytotoxicity Induced by Bothrops moojeni Snake Venom in Cultured Renal Tubular Epithelia. Toxicol Appl Pharmacol 181: 124-132.
-
Pathan J, Martin A, Chowdhury R, Chakrabarty D, Sarkar A (2015) Russell's viper venom affects regulation of small GTPases and causes nuclear damage. Toxicon 108: 216-225.
-
Conlon JM , Attoub S, Arafat H, Mechkarska M, Casewell RN, et al. (2013) Cytotoxic activities of [Ser49] phospholipase A2 from the venom of the saw-scaled vipers Echis ocellatus, Echis pyramidum leakeyi, Echis carinatus sochureki, and Echis coloratus. Toxicon 71: 96-104.
-
Ali G, Kak M, Kumar M, Bali S K, Tak SI, et al. (2004) Acute renal failure following echis carinatus (saw - scaled viper) envenomation. Indian J Nephrol 14: 177-181.
-
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3: 1125-1131.
-
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: Comparison of LDH, neutral red,MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160: 171-177.
-
Cornelis M, Dupont C, Wepierre J (1992) Prediction of eye irritancy potential of surfactants by cytotoxicity tests in vitro on cultures of human skin fibroblasts and keratinocytes. Toxicol In Vitro 6: 119-128.
-
Chiba K, Kawakami K, Tohyama K (1998) Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol In Vitro 12: 251-258.
-
Vian L, Vincent J, Maurin J, Fabre I, Giroux J, et al. (1995) Comparison of three in vitro cytotoxicity assays for estimating surfactant ocular irritation. Toxicol In Vitro 9: 185-190.
-
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516.
-
Park EK, Mak SK, Kültz D, Hammock BD (2008) Determination of cytotoxicity of nephrotoxins on murine and human kidney cell lines. J Environ Sci Health B 43: 71-74.
-
Wang SH, Shen XC, Yang GZ, Wu XF (2003) cDNA cloning and characterization of Agkistin, a new metalloproteinase from Agkistrodon halys. Biochem Biophys Res Commun 301: 298-303.
-
Smith SM, Wunder MB, Norris DA, Shellman YG (2011) A Simple Protocol for Using a LDH-Based Cytotoxicity Assay to Assess the Effects of Death and Growth Inhibition at the Same Time. PLoS ONE 6: 6.
-
Kendig DM, Tarloff JB (2007) Inactivation Of Lactate Dehydrogenase By Several Chemicals: Implications For In Vitro Toxicology Studies . Toxic In Vitro 21: 125-132.





