Clinical Medical
Reviews and Case Reports
Malignant Peritoneal Mesothelioma in a Clerk: A Diagnostic Dilemma
Victoria Achaval Rodriguez1#, Patricia Moreira1#, Maria Camara2#, Pilar Rondon1#, Maria Garrido3#, Ricardo Hitt3# and Maria Jose Echarri3#*
1Department of Internal Medicine, Hospital Universitario Severo Ochoa, Spain
2Department of Pathology, Hospital Universitario Severo Ochoa, Spain
3Medical Oncology Service, Hospital Universitario Severo Ochoa, Spain
#These authors contributed equally to this work
*Corresponding author: Maria Jose Echarri, Medical Oncology Service, Hospital Universitario Severo Ochoa, Leganes, Madrid, Spain, Tel: +34914818000 (ext. 8305), E-mail: mecharrigonzalez@gmail.com
Clin Med Rev Case Rep, CMRCR-3-127, (Volume 3, Issue 9), Case Report; ISSN: 2378-3656
Received: July 23, 2016 | Accepted: September 02, 2016 | Published: September 05, 2016
Citation: Rodriguez VA, Moreira P, Camara M, Rondon P, Garrido M, et al. (2016) Malignant Peritoneal Mesothelioma in a Clerk: A Diagnostic Dilemma. Clin Med Rev Case Rep 3:127. 10.23937/2378-3656/1410127
Copyright: © 2016 Rodriguez VA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Mesothelioma is a rare type of cancer originating from the surface linings of serous cavities; these membranes include the pleura, peritoneum, pericardium or tunica vaginalis testes [1].
Diffuse malignant peritoneal mesothelioma (DMPM) represents one-fourth of all mesotheliomas. Association of asbestos exposure with DMPM has been observed, especially in males. Incidence is increasing worldwide and is not expected to peak for another 5 to 20 years. The majority of patients present with abdominal pain and distension, caused by accumulation of tumors and ascitic fluid [1-3]. There is a less common form of MPM, which presents as a focal mass that does not spread through the peritoneal cavity called localized malignant peritoneal mesothelioma (LMPM). This latter form usually has a good prognosis once the lesion has been completely removed [4].
The three main cellular subtypes of peritoneal mesothelioma are epithelioid, sarcomatoid and biphasic [5]. There is a fourth subtype which includes rare types (desmoplastic, small cell, lymphohistiocytoid, deciduoid, and undifferentiated types). Of these subtypes, epithelioid mesothelioma is the most common [6].
The main difficulties in the pathologic diagnosis are differentiating a benign (reactive mesothelial hyperplasia) from malignant mesothelial proliferation, and the epithelioid subtype of MPM from adenocarcinoma metastatic in the serous membranes [7,8].
A definitive diagnosis of malignant mesothelioma usually requires an adequate biopsy with a concordant morphology and immunohistochemistry, in the context of appropriate clinical, radiologic and surgical findings.
Case Report
A 74-year-old male presented to the Emergency Department of our Hospital for abdominal distension and edema in lower limbs.
Two weeks before admission he developed progressive abdominal distension and edema in lower limbs, along with upper left quadrant pain. He reported a 10 kg loss in weight prior to a 5 kg gain over the three months leading up to presentation and he also associated anorexia.
The patient was a former smoker and had a history of hypertension. He had worked at an electrical appliance factory, as a sales manager. At presentation, the patient appeared cachectic, well-coloured and hydrated. There were no marks of chronic hepatopathy and no adenopathies. The abdomen was tense, distended and tender to the touch in the upper left quadrant. He had maleolar edema in both lower limbs. The remainder of the examination was normal.
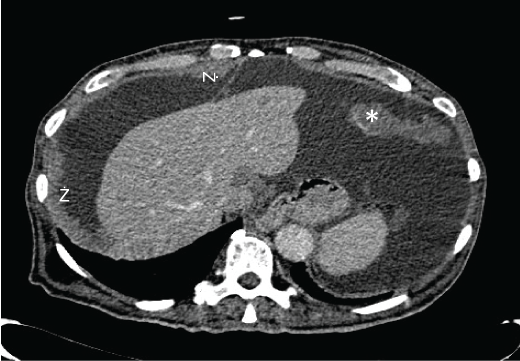
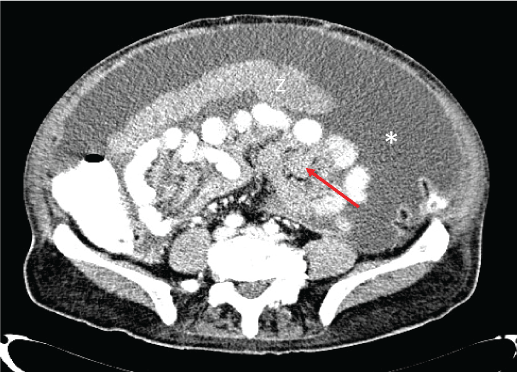
Basic blood chemical studies and renal- and liver-function tests were normal, as well as, carcinoembrionic antigen (CEA), fetoprotein α, CA19.9, CA 125 or PSA. A few days later a thoraco-abdomino-pelvic computed tomography (CT) was carried out. It showed abundant ascites and diffuse thickening of the peritoneum and multiple soft-tissue density areas on omentum and transverse mesocolon (Figure 1 and Figure 2). These findings suggested peritoneal carcinomatosis as a first diagnostic possibility. To complete the assessment an upper and lower endoscopy were done without abnormalities in either.
.
Figure 1: Abdomen CT: Nodular, irregular, thickened parietal peritoneum (arrowheads) and soft tissue omental mass (asterisk), congruent with peritoneal implants.
View Figure 1
.
Figure 2: Abdomen CT: diffuse ascites (asterisk), big soft tissue omental mass congruent with peritoneal implant (arrowhead) and thickened mesentery (red arrow).
View Figure 2
A paracentesis was performed to analize the ascitic fluid. This fluid was clear, the laboratory analisis showed an exudate with lymphocitic predominance and the cytology report showed no signs of malignancy. Finally a diagnostic laparoscopy was undertaken. In the laparoscopy, diffuse carcinomatosis was observed in parietal peritoneum, and there were some implants covering viscera. Multiple biopsies were collected.
.
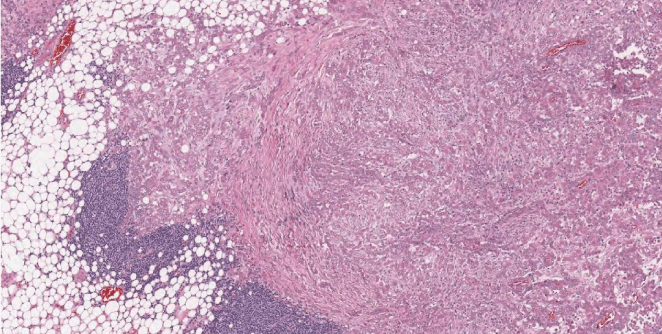
Figure 3: Histopathology (low magnification 10x): solid growth tumor invading the adipous tissue. Lymphoid aggregates are observed in the periferia.
View Figure 3
.
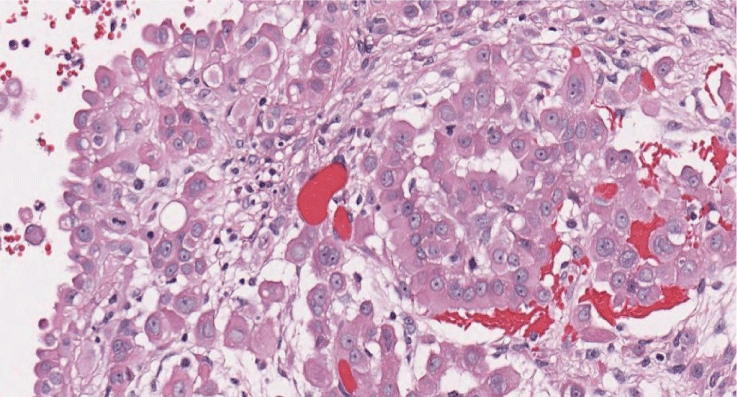
Figure 4: Histopathology (high magnification 40x) cells with abundant eosinophilic cytoplasm and round, vesicular nuclei with prominent nucleoli showing malignancy epitheliod proliferation.
View Figure 4
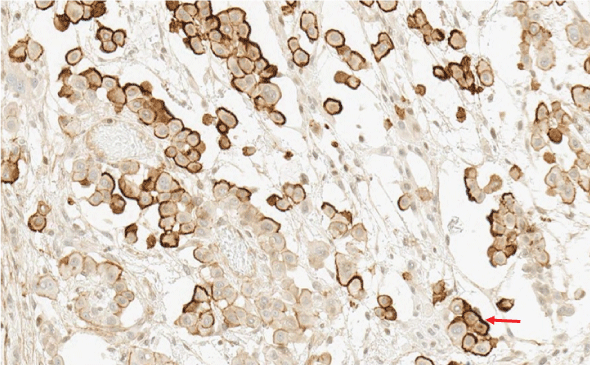
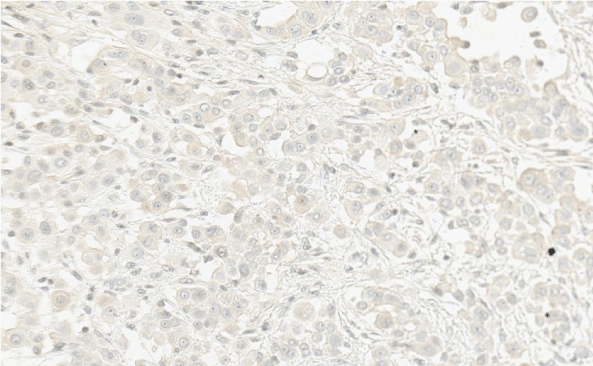
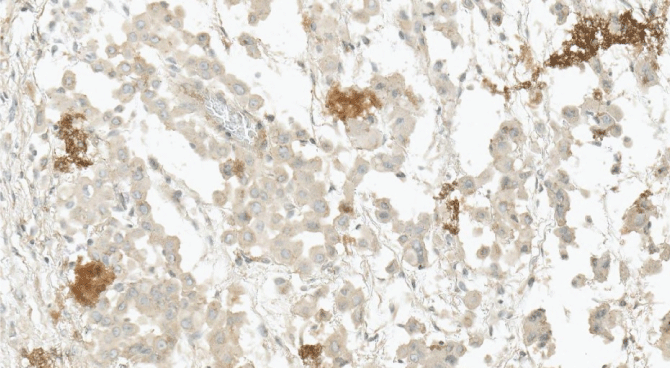
The histological report was: microscopic routine H-E (hematoxylin-eosin) stain revealed a solid pattern with numeorus sheets, nests and cords of round or poligonal cells that thoroughly invaded the conective and adipose tissue of the peritoneum. Cells showed an abundant eosinophilic cytoplasm and round, vesicular nuclei with prominent nucleoli. Mitotic figures were easy to find. The latter morphology was congruent with either adenocarcinoma or peritoneal mesothelioma (Figure 3 and Figure 4). The panel of inmmunohistochemical antibodies led to definitive diagnosis with HBME-1 (mesothelin), calretinin (Figure 5), CK5/6, CK7, D2-40 (podoplanin) (Figure 6) as positive markers and CEA (Figure 7), Ber-EP4 (Figure 8) as negative markers.
.
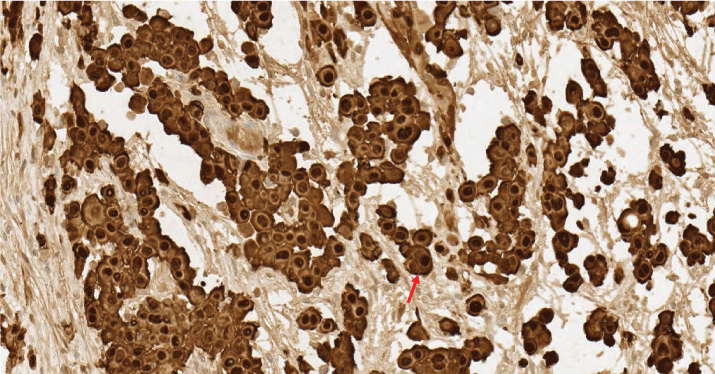
Figure 5: Intense and diffuse positive nuclear and citoplasmatic calretinin staining (red arrow).
View Figure 5
Before knowing the results of the immunohistochemical analysis, the patient was treated as an adenocarcinoma of unknown primary tumor according to Greco Criteria [9] with chemotherapy based on oxaliplatine and 5- fluorouracil . The definitive diagnosis came after the first cycle of chemotherapy and then the case was presented in the tumor board to considerer an extensive surgery including cytoreductivede bulking surgery and peritonectomy but the patient developed infectious complications (pneumonia) 17 days after the oncologic treatment and he declined progressively until his death, just a month after diagnosis.
Discussion
Malignant mesothelioma is a highly aggressive and rare neoplasm although the incidence is increasing worldwide [2]. After the pleural localization, the peritoneum mesothelioma is the second most frecuent type.
The diagnosis of malignant peritoneal mesothelioma (DMPM) is a challenge for clinicians due to the non-specific symptoms and signs of the disease (abdominal pain and distension, constitutional symptoms such weight loss or ascites) and for the pathologists due to the heterogeneity of histologic patterns which is the cause of delayed diagnosis [10]. The average time between the onset of symptoms and diagnosis was approximately 4.6 months [11].
As the disease progresses, they invariably die from intestinal obstruction or terminal starvation within a year, DMPMwas considered a pre-terminal condition, therefore attracted little attention [1]. In the last decades the association of asbestos exposure with DMPM has been observed, especially in males although this implication is less strong than in pleural mesotheliomas [12] there are some cases published [13,14]. As asbestos is an important occupational carcinogen Spain published a distribution of asbestos-related cancer cases by occupation from 1997 to 2011 [15]. These occupations included craft and related trades workers, plant and machine operators, assemblers and even clerks and sales workers. Our patientwas employedin an electrical appliance factory as a customer service clerk. Although his occupation was reflected in the anamnesis the suspicion of DMPM was not considered principally due to the few cases reported of asbestos-related cancer and clerksin Spain, in fact to our knowledge this is the first case reported of peritoneal mesothelioma in a clerk who was not directly exposed to asbestos. There is just a case reported of pleural mesothelioma [16] related to asbestos as a result of an “eyewitness” exposure. In the published case the patient was working as a clerk of an asbestos factory at least but in our case the exposure to the carcinogen was even remoter.
For the differential diagnosis of a patient with peritoneal carcinomatosis is necessary a thorough medical history and physical examination, basic blood and biochemistry analyses, CT of thorax, abdomen and pelvis and endoscopies guided by the symptoms of sign according with the Greco Criteria [17] and ESMO clinical practice of guidelines for diagnostic about cancer of unknown primary site [18]. The most frequent diagnosis is metastatic adenocarcinoma (digestive origin in males or gynecological serous papillary subtype in females) so the differential diagnosis between DMPM and peritoneal carcinomatosis (PC) of epithelial origin is crucial [19]. The laboratory findings can show elevated CA 125 in both entities though this marker cannot be used to confirm the diagnosis. In our patient this tumor marker was normal.
For the radiological assessment the CT scan is the most commonly used in cases of peritoneal carcinomatosis. There have been identified four radiologic features to distinguish DMPM from other peritoneal carcinomatosis [1]: diffuse involvement of all peritoneal surfaces, preponderance of disease in mid-abdomen and pelvic, presence of serous ascites rather than mucoid and absence of metastasis irrespective of the volume of disease. Although these findings may be helpful [20] in a trial of 95 patients (48 DMPM and 47 PC) there were no differences on the CT findings in terms of thickness, diameter of lymph nodes, ascites or viscera infiltration. They concluded that using a combination of CT findings may increase the ability to distinguish DMPM from PC [21]. In our case none of the radiographic findings were sufficiently specific to suspect a DMPM.
The definitive differential diagnosis comes from the histologic examination of tumor specimens [22]. In our patient the sample was obtained though a laparoscopy. The initial histological features with hematoxylin-eosin staining showed a solid pattern (which is the most common pattern of epithelioid peritoneal mesothelioma) of cells arranged in sheets, nests and cords. These characteristics can also be found in a reactive mesothelial hyperplasia and in metastatic adenocarcinomas [23] so the immunohistochemistry was necessary to a final diagnosis. Although there is not a specific marker for mesothelioma [24-26] there are many sensitive markers available in the diagnosis of epitheliod mesothelioma. Calretinin [27] was the first positive marker described for the diagnosis of mesothelioma specially to distinguish them from lung adenocarcinomas or renal cell carcinomas. Keratin 5/6 [23,24] is another positive marker for mesothelioma although its utility for distinguishing peritoneal mesotheliomas and serous carcinomas is low. Podoplanin [23,24] is a selective marker of lymphatic endothelium and frequently expressed in epithelioid mesotheliomas. Mesothelin is a positive marker for mesotheliomas but one-third of adenocarcinomas of the lung can expressed it so its utility is limited to discriminate both. Besides positive markers for mesothelioma it is useful to assess markers for carcinoma like MOC-31, Ber-EP4, CEA, BG-8 or p63 that are usually negative for mesotheliomas [23,24]. Our patient had four positive markers for mesothelioma (podoplanin, mesothelin, calretinin and CK5/6 and two negative markers to exclude a carcinoma (CEA and Ber-EP4). In a recent review it is recommended that two or more mesothelial markers have to be done to establish the diagnosis of DMPM [10]. If the results are concordant, the diagnosis may be considered established. If they are discordant, a second stage, expanding the panel of antibodies, may be needed. Other positive markers for mesothelima are WT-1 protein and thrombomodulin and for carcinomas TTF-1, estrogen or progesterone receptors, CA 19.9 or B 72.3 than can be useful in the diagnosis [24].
The prognosis of DMPM patients is poor with a median survival less than one year. Recently, several prospective trials have demonstrated a median survival of 40 to 90 months and 5-year survival of 30% to 60% after combined treatment using cytoreductive surgery (CRS) and perioperative intraperitoneal chemotherapy [1,28]. But the delay in the diagnostic process and the rapid progression of the disease make that the number of patients selected for CRS or intraperitoneal chemotherapy is low. So systemic chemotherapy is the most commonly use in patients with DMPM based on platinum and pemetrexed regimens [10]. Even in cases like in ours the necessity of an urgent treatment makes that we have to treat these patients without a definitive diagnosis according with the Greco Criteria of cancer of unknown primary site mentioned above [9,17]. In our case we treated the patient as a man with peritoneal carcinomatosis with adenocarcinoma histology before getting the immunohistochemistry with oxaliplatin and 5-FU. After one cycle we had the diagnosis of DMPM and a treatment with pemetrexed and carboplatin was scheduled but unfortunately his clinical situation was getting worse and the treatment was not possible. There are new targeted therapies under evaluation for DMPM like epidermal growth factor receptor (EGFR) inhibitors, phosphatidylinositol-3-kinase and mammalian target of rapamycin (PI3K/mTOR) inhibitors with few data and a promising line of investigation in the immunotherapy area [10,28].
Conclusion
Peritoneal mesothelioma is a rare and fatal tumor which diagnosis is usually delayed for non-specific signs or symptoms, histological heterogeneity and the need for invasive diagnostic tests. The patient we reported had an epithelial subtype of DMPM and for the differential diagnosis of metastatic adenocarcinoma the histopathological examination and the specific immunohistochemical stain were the keys to reach the diagnostic. In the era of molecular and genetic testing in oncology for diagnosis, especially for unknown primary tumors the clinical orientation, the CT findings and the immunohistochemistry are still mandatory but if we want to offer our patients the best therapeutic choices that include surgery, intraperitoneal chemotherapy or systemic chemotherapy the diagnostic process has to be rapid.
DMPM is an occupational disease related to asbestos exposure and medical professionals should take in account this possibility among patients with previous asbestos contact. Our patient has an indirect contact to asbestos being this relationship an exceptional cause of disease. Probably the risk of developing mesothelioma among individuals who are not directly working with asbestos is very low but clinicians should be aware of this can happen and we recommend the differential diagnosis of peritoneal mesothelioma in patients with peritoneal carcinomatosis even if they had any of the rare occupational professions related to asbestos. The guidelines for diagnosis of cancer unknown primary site could include this differential diagnostic between carcinoma and mesothelioma.
Conflicts of Interest
No financial disclosures to report of any author.
References
-
Munkholm-Larsen S, Cao CQ, Yan TD (2009) Malignant peritoneal mesothelioma. World J Gastrointest Surg 1: 38-48.
-
Robinson BW, Lake RA (2005) Advances in malignant mesothelioma. N Engl J Med 353: 1591-1603.
-
Hong S, Bi MM, Zhao PW, Wang XU, Kong QY, et al. (2016) Malignant peritoneal mesothelioma in a patient with intestinal fistula, incisional hernia and abdominal infection: A case report. Oncol Lett 11: 2047-2050.
-
Levy AD, Arnaiz J, Shaw JC, Sobin LH (2008) From the archives of the AFIP: Primary Peritoneal Tumors: Imaging Features with Pathologic Correlation. Radiographics 28: 583-607.
-
Ordonez NG (2007) Pathologic characterization and differential diagnosis of malignant peritoneal mesothelioma. Recent Results Cancer Res 169: 123-136.
-
http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/peritoneum-15protocol-3201.pdf.
-
Krasuski P, Poniecka A, Gal E (2002) The diagnostic challenge of peritoneal mesothelioma. Arch GynecolObstet 266: 130-132.
-
Churg A, Galateau-Salle F (2012) The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med 136: 1217-1226.
-
Greco FA, Hainsworth JD (2011) Cancer of unknown primary site. In: DeVita VT, Lawrence TS, Rosenberg SA, DeVita, Hellman and Rosenberg’s Cancer: Principles and practice of Oncology. (9th edn), Lippincott Wiliams and Wilkins, Philadelphia, 2033-2051.
-
Alexander HR Jr, Burke AP (2016) Diagnosis and management of patients with malignant peritoneal mesothelioma. J Gastrointest Oncol 7: 79-86.
-
Kaya H, Sezgi C, Tanrikulu AC, Taylan M, Abakay O, et al. (2014) Prognostic factors influencing survival in 35 patients with malignant peritoneal mesothelioma. Neoplasma 61: 433-438.
-
Torrejon Reyes PN, Frisancho O, Gomez A, Yabar A (2010) Malignant peritoneal mesothelioma. Rev Gastroenterol Peru 30: 82-87.
-
Fonte R, Gambettino S, Melazzini M, Scelsi M, Zanon C, et al. (2004) Asbestos-induced peritoneal mesothelioma in a construction worker. Environ Health Perspect 112: 616-619.
-
Candura SM, Boeri R, Teragni C, Chen Y, Scafa F (2016) Renal cell carcinoma and malignant peritoneal mesothelioma after occupational asbestos exposure: case report. Med Lav 107: 172-177.
-
Garcia-Gomez M, Menendez-Navarro A, Lopez RC (2015) Asbestos-related occupational cancers compensated under the Spanish National Insurance System, 1978-2011. Int J Occup Environ Health 21: 31-39.
-
Chia SE, Lee HS (1990) Malignant mesothelioma in a clerk working in an asbestos factory. Ann Acad Med Singapore 19: 380-381.
-
Greco FA (2013) Cancer of unknown primary site: improved patient management with molecular and immunohistochemical diagnosis. Am Soc Clin Oncol Educ Book 175-181.
-
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, et al. (2015) Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26: 133-138.
-
Bretagne CH, Petitjean A, Felix S, Bedgedjian I, Algros MP, et al. (2016) Metastasis revealing malignant peritoneum mesothelioma: About the difficulty to identify the primary tumors. Ann Pathol 36: 105-110.
-
Kato K, Gemba K, Fujimoto N, Aoe K, Takeshima Y, et al. (2016) Computed Tomographic Features of Malignant Peritoneal Mesothelioma. Anticancer Res 36: 1067-1072.
-
Liang YF, Zheng GQ, Chen YF, Song H, Yin WJ, et.al. (2016) CT differentiation of diffuse malignant peritoneal mesothelioma and peritoneal carcinomatosis. J Gastroenterol Hepatol 31: 709-715.
-
Patel NP, Taylor CA, Levine EA, Trupiano JK, Geisinger KR (2007) Cytomorphologic features of primary peritoneal mesothelioma in effusion, washing, and fine-needle aspiration biopsy specimens: examination of 49 cases at one institution, including post-intraperitoneal hyperthermic chemotherapy findings. Am J Clin Pathol 128: 414-422.
-
Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, et al. (2013) Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 137: 647-667.
-
Ordonez NG (2006) The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol 19: 34-48.
-
Ordonez NG (2007) What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol 38: 1-16.
-
Kawai T, Tominaga S, Hiroi S, Ogata S, Nakanishi K, et al. (2016) Peritoneal malignant mesothelioma (PMM), and primary peritoneal serous carcinoma (PPSC) and reactive mesothelial hyperplasia (RMH) of the peritoneum. Immunohistochemical and fluorescence in situ hybridisation (FISH) analyses. J ClinPathol 69: 706-712.
-
Doglioni C, Dei Tos AP, Laurino L, Iuzzolino P, Chiarelli C, et al. (1996) Calretinin: a novel immunocytochemical marker for mesothelioma. Am J SurgPathol 20: 1037-1046.
-
Cao S, Jin S, Cao J, Shen J, Hu J, et al. (2015) Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis 30: 1-10.